INTRODUCTION The facial nerve (VII skull pair) is responsible for mimics muscle inervation, for tonus at rest and for voluntary and involuntary contraction of each hemiface(1). Such muscles are responsible for facial movements and for human emotion expression(2).
Facial nerve lesions may produce deformities in variable degrees, causing aesthetic and functional disorders in patients. The main causes of facial paralysis include cerebrovascular accidents, surgical and traumatic lesions and paralysis of non-determined etiology, which is the most frequent one. The other causes include nervous and muscular alterations, viral and bacterial infections and development anomalies, which correspond to lower percentage of facial paralysis etiology(3). Patients with facial paralysis present common characteristics. The paralyzed side has few wrinkles, a less evident nasolabial groove and fall of labial comissure and of eyebrow. The contralateral side responds with muscular hyperkinetic reaction due to lack of tonus in the paralyzed side. Such vetorial force unbalance creates facial deviations which are observed when patient is at rest and mainly at smiling.
The facial paralysis treatment is complex. After the acute phase, the treatment includes nerve grafts, muscular transferences, microsurgical flaps, neurectomies, miotomies, that is, multiple ways of treatment which can not provide the patient with the desirable face balance(4,5,6,7,8). In addition to those methods, the use of skin suspension or resection such as ritidoplasty, blefaroplasty, lagoftalmy correction is essential to improve the aesthetic result(9,10,11).
Even after those clinical-surgical therapies, it is verified that the patients still present observable face asymmetry in static and dynamic positions due to muscle hyperkinesias which is contralateral to the paralysed side. Besides the surgical methods to control hyperkinesias, there is the possibility to use botulinium toxin to improve face asymmetry on patients who have facial paralysis(12).
This study aims at evaluating the efficiency and security of A kind botulinium toxin application in the reduction of muscular hyperkinesias in the perioral region which is contralateral to facial paralysis.
METHODThis study has been approved by the Comitê de Ética do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Ethics Comittee of São Paulo University Medicine College) on September 10, 2003, under the number 690/03.
Among the inclusion criteria, there were patients of both genders, between 15 and 70 years old, with facial paralysis of any etiology, with more than one year of evolution and which presented facial muscular hyperkinesis which was contralateral do the paralysed side.
The exclusion criteria include patients with previous treatment using botulinium toxin, with neuromuscular diseases in motor plates, using aminoglycoside antibiotics, pregnant or breastfeeding women, or people holding systemic pathologies.
Eighteen patients (12 women and 6 men), aging an average of 40.3 years (17-70 years), holding unilateral facial paralysis for more than one year of evolution, were included in this study. The etiology of facial paralysis include for cases with non-determined origin, 8 tumoral, 1 congenital and 5 facial nerve traumatic lesions (1 running over, 1 fall from height, 1 aggression and 2 gun wounds).
The patients were injected with Botulinium Toxin of A kind (Dysport
® - Ipsen Limited, Berkshire, England - 500U), reconstituted with 4 ml of sodium chloride 0.9% with final concentration of 12.5U per 0.1 ml ressuspended solution. The drug has been applied in studied areas through intramuscular via with 30 G 1/2 needle in 1 ml syringe, in order to guarantee applied volume accuracy. The application has been performed at a 45º angle to skin, with the patient at dorsal recumbent. Contact with periosteum has been avoided. The dosis of Dysport
® (Ipsen Limited, Berkshire, England) and the locations of injections have been determined according to pilot study.
Each patient has been submitted to the application of 0.9 ml of botulinium toxin suspension, adding up 112.5 units. The muscles, the location of application, units and corresponding volume are described as follows: 1) Mayor Zigomatic Muscle in its origin: 12.5 U (0.1 ml); 2) Minor Zigomatic Muscle in its origin: 6.25 U (0.05 ml); 3) Upper Lip Elevator Muscle at the orbit floor level: 6,25 U (0,05 ml); 4) Upper Lip Elevator Muscle and Nose Wing Elevator Muscle at nose wing level: 6.25 U (0.05 ml); 5) Risorious Muscle at 2 cm from labial comissure: 25 U (0.2 ml); 6) Modiulus at 0.5 cm from labial comissure: 12.5 U (0.1 ml); 7) Depressure Anguli Oris Muscle at 0.5 from labial comissure: 25 U (0.2 ml); 8) Depressure Lower Lip Muscle at 1.0 cm mucocutaneous transition: 18.75 U (0.15 ml) (Picture 1).
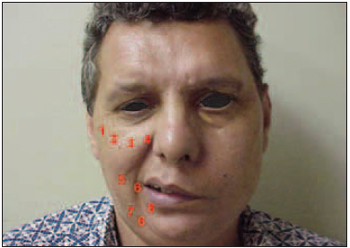
Picture 1. Botulinium Toxin Application Points.
Patients were submitted to subjective and objective evaluation 14, 28, 42, 56, 90, 150 and 180 days after Dysport
® injection (Ipsen Limited, Berkshire, England).
The objective evaluation (quantitative) included static evaluation (at rest) and dynamic evaluation (maximum contraction at smiling) in the perioral region contralateral to facial paralysis. The quantitative measurings were performed by three evaluators, with a digital paquimeter (Mitutoyo, model MIP/E code number 500-143B; resolution 0.01 mm/nominal capacity: 150 mm/accuracy: margin of error: +/- 00.02 mm, repetitiveness: 0.01 mm) in three axis x, y, and z, and the average has been used as a reference value. The photographic documentation has been performed with a digital camera with a camcorder (SONY
® Digital Mavica - MVC-FD 91), which also allowed the animated registration (two photos per position have been taken).
For the X axis, the measuring X1 (static), corresponded to the distance between the endocanthion to the cheilion and the measuring X2 (dynamic) corresponded to the same distance when the patient smiled at maximum contraction. For the Y axis, the measuring Y1 (static), corresponded to the distance between the enxocanthion to the cheilion and the measuring Y2 (dynamic) corresponded to the same distance when the patient smiled at maximum contraction. For the Z axis, the measuring Z1 (static), corresponded to the distance between the tragion to the cheilion and the measuring Z2 (dynamic) corresponded to the same distance when the patient smiled at maximum contraction. The difference between each static and dynamic measuring (" > in mm) has been analysed through a curve.
The subjective evaluations have been performed by the patients in all visits. The degree of improvement in appearance has been analysed using the following scale: -1= worsening, 0= absence of improvement, 1= mild improvement, 2= moderate improvement, 3= great improvement. The degree of patient's satisfaction has been according to the following criteria: unsatisfied (0), satisfied (1) or very satisfied (2). The following statistical tests have been used: the Hartley test, to verify variable homogeneity and further variable analysis with repeated measuring in a factor (ANOVA), as well as the Newman-Keuls test for multiple comparisons. The action percentage loss has been calculated with the following formula: {(1 - difference of static and dynamic meausuring on Day 0 / difference of static and dynamic meausuring on Day 14 until Day 180)*100}.
The adverse events have been verified in all visits of this study. Pain during application followed an analogical scale from 0 to 10, in which 0 meant total absence of pain and 10 meant unbearable pain.
RESULTS
Objective Analysis
X Measuring Before the application, the average difference between the dynamic and static measuring for the X axis was 4.5 mm (±1.86 mm). The best result for the X axis has been observed on the 28th day (2.35 mm ± 1.45 mm). During the action time there was statistic significance (ANOVA, p<0.00001). Multiple comparisons revealed significant statistical differences between the measuring on day ) and on the 14th day (p=0.00023), 28th (p=0.00014), 42nd (p=0.00044), 56th (p=0.00044), 90th (p=0.00037), e 150th (p=0.0037). The difference between the static measuring and the dynamic measuring on X axis can be verified on Picture 2.
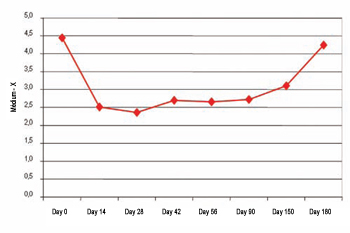
Picture 2. Difference between static and dynamic (X measuring).
The difference of static and dynamic measuring versus the difference of static and dynamic measuring on day 0 for the X measuring has demonstrated that the highest average has been obtained on the 28th day (46.86 ± 34.25). Consequently, being the maximum action peak considered on the 28th day (100% of the action), action percentage loss has been 26.6% on 42nd day, 26% on 56th day, 26.2% on 90th day, 38.9% on 150th day, and 94.7% on 180th day.
Y Measuring Before the injection, the average difference between the static and dynamic measuring for the Y axis was 8.07 mm (±3.23 mm). The lowest difference has been obtained on the 42nd day (3.81 mm ± 2.35 mm). During the action time there was statistic significance (ANOVA, p<0.00001). Multiple comparisons revealed significant statistical differences between the measuring on day 0 and on the 14th day (p=0.00012), 28th (p=0.00012), 42nd (p=0.00012), 56th (p=0.00014), 90th (p=0.00011), e 150th (p=0.0039). The difference between the static measuring and the dynamic measuring on Y axis can be verified on Picture 3.
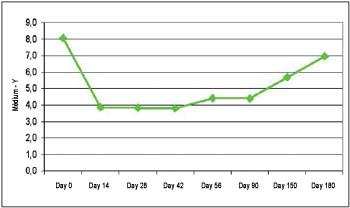
Picture 3. Difference between static and dynamic (Y measuring).
The difference of static and dynamic measuring versus the difference of static and dynamic measuring on day 0 for the Y measuring has demonstrated that the highest average has been obtained on the 14th day (52.87 ± 20.09). Consequently, the maximum action peak has been on the 14th day (100% of the action), action percentage loss has been 3.4% on 28th day, 15.7% on 42nd day, 19.9% on the 56th day, 19.1% on 90th day, 55.8% on 150th day, and 84% on 180th day.
Z Measuring Before the injection, the average difference between the static and dynamic measuring for the Z axis was 12.88 mm (±4.40 mm). The lowest difference has been obtained on the 42nd day (5.49 mm ± 2.44 mm). During the action time there was statistic significance (ANOVA, p<0.00001). Multiple comparisons revealed significant statistical differences between the measuring on day 0 and on the 14th day (p=0.00014), 28th (p=0.00012), 42nd (p=0.00012), 56th (p=0.00012), 90th (p=0.00011), e 150th (p=0.0012). The difference between the static measuring and the dynamic measuring on Z axis can be verified on Picture 4.
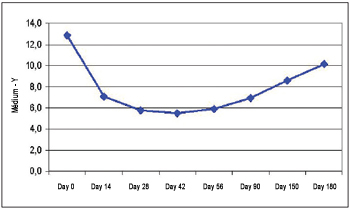
Picture 4. Difference between static and dynamic (Z measuring).
The difference of static and dynamic measuring versus the difference of static and dynamic measuring on day 0 for the Z measuring has demonstrated that the highest average has been obtained on the 42nd day (55.10 ± 19.79). Considering that the best result has been obtained on the 42nd day (100% of the action), action percentage loss has been 4.9% on 56th day, 23.2% on 90th day, 45% on 150th day, and 69.7% on 180th day.
Subjective Analysis
Patients Satisfaction In terms of patients' satisfaction, 17 out of 18 patients (94%) reported that they have been satisfied or very satisfied with their results on the 14th day. All patients, 18 out of 18 (100%) reported that they have been satisfied or very satisfied with their results on the 28th, 42nd, 56th and 90th days. There has been a reduction in the 94% percentage (17 out of 18) on the 150th day to 78% (14 out of 18) to the 180th day. The patient satisfaction degree may be observed on Picture 5.
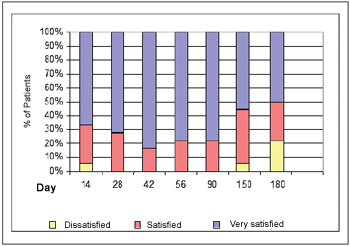
Picture 5. Satisfaction degree with the treatment.
The improvement in aesthetic appearance has been unanimous. On the 14th day, 72% of the patients (13/18) reported mild improvement or great improvement. On the 28th, 42nd, 56th and 90th days, 100% of the patients reported mild or great improvement and such percentage dropped to 72% on the 150th day and to 56% on the 180th day. The patients' improvement degree may be observed on Picture 6.
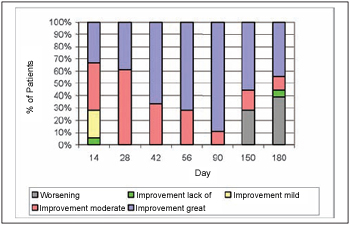
Picture 6. Aesthetic improvement degree.
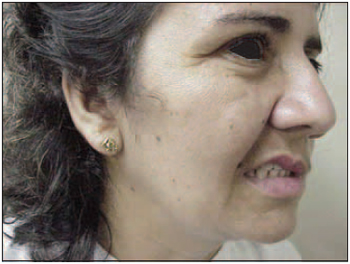
Pictures 7 (a and b) and 8 (a and b) demonstrate clinical cases on the botulinium toxin pre-application and post-application phases.
7.a. Pre-treatment: Excessive muscle force elevator and lateral muscles of prioral área.

7.b. Post-treatment: Dynamic Balance after application of botulinium toxin at smiling.

8.a. Patient before the treatment presenting important deviation in the right hyperkinetic hemiface with ocular closing, profound nasogenian groove and mouth distortion.

8.b. After the treatment, facial balance has been obtained without pathology stigma. The patient reported self-steem improvement and social reintegration.
On the botulinium toxin application day, pain evaluation during DYSPORT injection demonstrated an average of 3.15 in a scale from 0 to 10 (previously decribed).
On the 14th day, the following adverse events have been verified: mild difficulty to drink without liquid loss (9/18); mild difficulty or pain to speak (5/18); mild difficulty to chew without oral incontinence for solids (5/18); mild difficulty to kiss, to put on lipstick or to pout (3/18); hearing alteration (2/18) and mild difficulty to swallow (1/18).
The complaints progressively diminished during the following visits, and on the 90th day there has been total absence of complaints in most of the cases, except in two patients.
DISCUSSION The rehabilitation of patients with facial paralysis aims at recovering the symmetry at rest and during voluntary and involuntary facial mimics. The surgical methods for facial paralysis treatment include excisions, grafts, weight and suspenders implants, passive and active supports, muscular transposition and nerve and muscle free transplants (3,4,7,13).
The hyperkinesis surgical correction procedures produce aesthetic sequels such as: scars, hypoesthesia, hyperesthesia, paresthesia, partial paresis, asymmetry, incomplete correction and recurrences (26). Eventually sincinesis which complicate patient's rehabilitation even more may appear (14,15). Usually, the procedures are multiple and involve several hospitalizations. The best results are obtained through the association of techniques. Besides neurectomy and miectomy, several other techniques such as ritidoplasty, blefaroplasty and lagoftalmo correction should be considered, in addition to the use of botulinium toxin, as a muscle action inhibitor(9,1011,16,17,18).
The botulinium toxin provides reversible chemical muscle inhibition, and this way it can be used as a therapeutical test before definitely changing the muscle function through neurectomies and miectomies. It presents inclusive potential to treat face hypertony which brings anasthomosis between the facial and hypoglossal nerves. Furthermore, it can improve asymmetry (18,19,20,21,22). It is the chosen treatment for the motor and autonomic effects originated from aberrant nervous regeneration (23,24).
The studies of botulinium toxin application in facial paralysis present high levels of success in symptoms temporary relief(12), no matter what their etiology is. The critics on botulinium toxin treatment is related to its temporary effect in patients with facial paralysis, and because of that some authors still prefer the surgical methods. However, we disagree with this position, once some therapies should be complementary, not excluding.
The application of botulinium toxin of A kind should be considered an ambulatory procedure, without the need of local anesthesia(17,25). In this study, there has not been any similar complication to surgical complications and the pain evaluation during the application presented an average of 3.15 over 10 (being 10 an unbearable pain), being thus considered very bearable by the patients.
The adverse effects of botulinium toxin use are rare(27). In this study, there has not been any precocious complication such as hematomas, infections or allergies, not even late complications such as definitive oral incontinence for solids and liquids, permanent hearing, chewing and speech difficulties. With the abrupt muscle function modification, 50% of the patients reported mild difficulty to drink in the first evaluation after the injection, but without liquid loss or oral incontinence which would harm such function. There has been mild difficulty to chew in three patients, but there has not been food loss through the mouth during meals. Five patients presented mild difficulty or pain at speaking, probably due to excessive muscle use and to muscle contraction pattern.
All patients have reported that modification intensity after applications has been mild or discrete. The applied doses, hyperkinesis and paralysis severity may have direct influence over the presence of adverse effects.
We have evaluated aesthetic improvement or gain and patients' satisfaction with the treatment. Despite the adverse effects, there have been high levels in satisfaction and aesthetic improvement evaluation of all patients. We agree with Mountain(15) when he says that complications are not lasting and that there is not any systemic effect with botulinium toxin application. The hyperkinesis treatment with botulinium toxin may reach even better results if the treatment is associated to speech therapy and physiotherapy.
All patients have presented facial asymmetry improvement after the botulinium toxin application, which could be proved through the objective evaluation during the aesthetic and dynamic analysis. There has been significant reduction of dynamic asymmetry, which has been verified through the curves of X2, Y2 and Z2 axis. The botulinium toxin has also interfered in the static muscle tonus and brought relaxation with correction or reduction of static deviations (X1, Y1, Z1). There has been reduction of muscle hyperkinesis which has been verified through the approach of dynamic axix to static axis in a statistically significant way. The observed aesthetic benefit has been the reduction of mouth deformity at smiling.
In relation to the used doses (112.5 U), we believe it has been low for some patients, not totally reducing the hyperkinesis. In other patients, it may have been excessive, bringing severe weakening or even muscle hypotony and higher presence of adverse events.
Bikhazi NB and Maas CS (29) report that the effect lasts 3 months when blocking 4 muscles at the contralateral side. Our study has demonstrated longer duration, around 6 months through blocking contralateral muscles in 8 places.
The botulinium toxin used to treat patients with facial paralysis may be considered indispensable resource for professionals who deal with this kind of lesion. We agree with Clark (28) when he says that the botulinium toxin may be considered the main treatment for definitive or temporary asymmetries in patients with facial paralysis.
However, the most important fact is the application's potential on children and adolescents who, during the bone and muscle development phase, would have great benefit, if further deformities originated from facial paralysis could be avoided. We will be surely producing minor functional and aesthetic sequels in our patients.
CONCLUSIONS The results obtained with the use of botulinium toxin have made us conclude that there has been significant reduction in muscle hyperkinesis contralateral to facial paralysis proved by objective analysis; there has been improvement of the global aesthetic aspect verified by the patients through subjective analysis; the adverse events were of mild intensity and not lasting; there has been high degree of satisfaction with the treatment, reported by most of the patients.
REFERENCES1. Aviv JE, Urken ML. Management of the paralyzed face with microneurovascular free muscle transfer. Arch Otolaryngol Head Neck Surg. 1992;118:909-12.
2. de Maio M, Soares MFD. Aplicação de toxina botulínica em paralisia facial. In: de Maio M. Tratado de medicina estética. São Paulo: Roca; 2004. p.1361-71.
3. Bento RF, Brito RV. Gunshot wounds to the facial nerve. Otol Neurotol. 2004;25(6):1009-13.
4. Ueda K, Hari K, Asato H, Yoshimura K, Yamada A. Evaluation of muscle graft using facial nerve on the affected side as a motor source in the treatment of facial paralysis. Scand J Plast Reconstr Surg Hand Surg. 1999;33(1):47-57.
5. Terzis JK, Kalantarian B. Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation. Plast Reconstr Surg. 2000;105(6):1917-31.
6. Kermer C, Millesi W, Paternostro T, Nuhr M. Muscle-nervemuscle neurotization of the orbicularis oris muscle. J Craniomaxillofac Surg. 2001;29(5):302-6.
7. Bento RF, Almeida ER, Miniti A. Anastomosis of intratemporal facial nerve with fibrin tissue adhesive. Eur Arch Otorynolaringol. 1994:S387-S388.
8. Benardes DFF, Gomez MVS, Pirana S, Bento RF. Functional profile in patients with facial paralysis treated in a myofunctional approach. Pro Fono. 2004;16:151.
9. Choo PH, Carter SR, Seiff SR. Upper eyelid gold weight implantation in the asian patient with facial paralysis. Plast Reconstr Surg. 2000;105:855-9.
10. Adant JP. Endoscopically assisted suspension in facial palsy. Plast Reconstr Surg. 1998;102(1):178-81.
11. Shumrick KA, Pensak ML. Early perioperative use of polytef suspension for the management of facial paralysis after extirpative skull base surgery. Arch Facial Plast Surg. 2000;2(4):243-8.
12. Neuenschwander MC, Pribitkin EA, Sataloff RT. Botulinum toxin in otoryngology: a review of its actions and opportunity for use. Ear Nose Throat J. 2000;79(10):788-9.
13. Seeley BM, Wyatt CT, Papay FA. A multivectored boneanchored system for facial resuspension in patients with facial paralysis. Plast Reconstr Surg. 2001;108(6):1686-91.
14. Guerrissi JO. Selective myectomy for postparetic facial synkinesis. Plast Reconstr Surg. 1991;87(3):459-66.
15. Mountain RE, Murray JA, Quaba A. Management of facial synkenesis with clostridium botulinum toxin injection. Clin Otolaryngol. 1992;17(3):223-4.
16.Fagien S. Temporary management of upper lid ptosis, lid malposition and eyelid fissure asymmetry with botulinum toxin type A. Plast Reconstr Surg. 2004;114(7):1892-902.
17.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21(12):1740-7.
18. de Maio M. Use of botulinum toxin in facial paralysis. J Cosmet Laser Ther. 2003;5(3-4):216-7.
19. Finn JC. Botulinum toxin type A: fine-tuning treatment of facial nerve injury. J Drugs Dermatol. 2004;3(2):133-7.
20. Bulstrode NW, Harrison DH. The phenomenon of the late recovered Bell's palsy: treatment optins to improve facial symmetry. Plast Reconstr Surg. 2005;115(6):1466-71.
21. Krohel GB, Cipollo CL, Gaddipati K. Contralateral botuninum injections improve drinking ability and facial symmetry in patients with paralysis. Am J Opthalmol. 2005;139(3):540.
22. Blitzer A, Sulica L. Botulinum toxin: basic science and clinical uses in otolaryngology. Laryngoscope. 2001;111:218-26.
23. Boroojerdi B, Ferbert A, Schwarz M, Herath H, Noth J. Botulinum toxin treatment of synkinesia and hyperlacrimation after facial palsy. J Neurol Neurosurg Psychiatry. 1998;65(1):111-4.
24. Riemann R, Pfennigsdorf S, Riemann E, Naumann M. Succesful treatment of crocodile tears by injection of botulinum toxin into the lacrimal gland: a case report. Ophtalmology. 1999;106(12):2322-4.
25. Carruthers A, Caruthers J. Botulinum toxin type A: history and current cosmetic use in the upper face. Semin Cutan Med Surg. 2001;20(2):71-84.
26. Dobie RA, Fisch U. Primary and revision surgery (selective neurectomy) for facial hyperkinesia. Arch Otorynolaringol Head Neck Surg. 1986;112(2):154-63.
27. Armstrong MW, Mountain RE, Murray JA. Treatment of facial synkinesis and facial asymmetry with botulinum toxin type A following facial nerve palsy. Clin Otolaryngol. 1996;21(1):15-20.
28. Clark RP, Berris CE. Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis. Plast Reconstr Surg. 2005;115(2):573-4.
29. Bikhazi NB, Maas CS. Refinement in the rehabilitation of the paralyzed face using botulinum toxin.Otolaryngol Head Neck Surg. 1997; 117(4):303-7.
1. Master's Degree In Medicine and Doctor's Degree In Science From FMUSP (São Paulo Medical College) (Member of Brazilian Society of Plastic Surgery, Member of The International Society of Aesthetic Plastic Surgery.)
2. Currently Taking Doctor's Degree on Plastic Surgery From FMUSP. (Member of Brazilian Society of Plastic Surgery)
Institution: FMUSP - Faculty of Medicine of the University Sao Paulo
Mauricio de Maio
Mailing address: Ibirapuera Avenue, 2907 - Conj. 1202 - Moema - CEP: 04029-200 - Sao Paulo/SP - Phone/Fax: (11) 5535-9286 - E-mail: mauriciodemaio@uol.com.br
CNPQ
This article was submitted to Publication Management System from RAIO on October 11, 2006 and was approved on December 20, 2006 22:30:22.