INTRODUCTIONOtopathology, a study of temporal bone through histological cuts, consist of an area that was stimulated especially after the World War II, when collections of temporal bones in Europe and USA were expressively expanded. Therefore, there are not, even nowadays, laboratories which process temporal bone to histological cuts as a routine. This is due to the complexity degree of the process and to necessary expense in order to obtain good quality specimen to be studied. So, it is important a revision on some basic topics of structure and ultra-structure from inner ear.
The target of this article is to describe collected information about structure and ultra-structure of inner ear from a tipical mammalian. Although initial studies were performed on humans, much information came from studies with rodents, and more recently from cats and dogs. Comparing findings among specimens1, it is possible to establish some structure and organization similarities of cochlea. The presented infomormation form a summary of this proximity, so, qualitative and quantitative differences are not emphasized. Despite the fact that this revision deals with different components from the inner ear in a separate way, one might not forget that these structures work together in order to develop hearing and vestibular function. Most of recent researches are based on nature of theses interactions and on how morphological laterations are related with changes on function.
LITERATURE REVISIONCochlea inside inner ear holds reponsible cells by sound perception and it presents a snail shape. Unfortunately, to researchers, this organ is in the interior of temporal bone, which is a bone structure highly mineralized, dense and difficult to be accessed. Regarding these difficulties, microscopic anatomy and cochlea histology were described around 19th century by Retzius, Huschke, Reissner, Kolliker, Deiters, Hensen and Corti. According to their studies, the cochlea is composed by a bone labyrinth, inside which it is found a cell structure that is membranous labyrinth. Such detais are easily seen in a histological section of parallel cochlea at its larger axis (Picture 1).
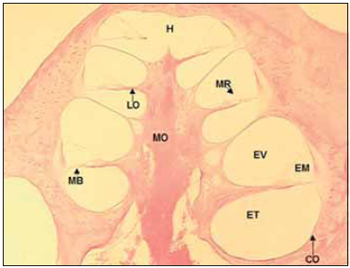
Picture 1. Fotomicrografia a section of 20µ médiomodiolar. Cóclea left of a dog. Colouring with hematoxylin and eosin, 40x. CO = Capsule optics, MO = Modiolo, EV = Vestibular Scale, EM = Scale average, MR = Membrane of Reissner, ET = Scale tympanic, MB = basilar membrane, LO = spiral blade bone, H = Helicotrema.
In the bone labyrinth is the optical capsule, external bone limit of cochlea and modiolus, bone tube that forms central axis of the cochlea. The bone labyrinth is divided in three microcompartment or scales forming a spiral around modiolus, from the base to the vertex of the cochlea. The apical compartment - vestibular scale (VE) - is separated from medium scale (ME) by Reissner Membrane. Medium scale separated from tympanic scale (TE) - inferior compartiment - by basilar membrane (MB), over which cells of Cortu organ rest, and by bone spiral lamina. Vestibular and tympanic scales communicate by a small opening in the cochlear vertex called helicotrema (H). Tympanic scale ends in the base of chochlea in the round window, which is coverd by a fine memebrane over optical capsule (Picture 1).
Tympanic and vestibular scales form a perilynphatic space with a fluid rich in sodium called perilymph, while medium scale forms the endolymphatic space composed by a fluid rich in potassium - endolymph. It was belived in the past that the limits between spaces referred to to membranes that limites such areas. Therfore, the terms 'cochlea canal' and 'endolymphatic space' form delimited that includes Reissner membrane, basilar membrane, spiral edge, stria vascularis and spiral ligament. It is know that limits between scales are not necessarily the same as the ones that delimit fluids. For instance, structures that separate endolymph are the 'tight junction' between adjancent epithelial cells. Such cell junctions block free diffusion of the endolymph between epithelial cells (Picture 2).
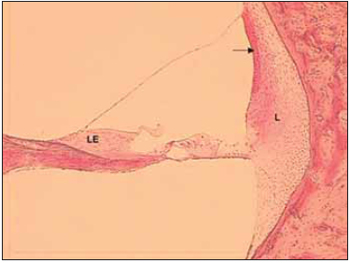
Picture 2. Fotomicrografia a section of 20µ through one half of a cochlear spin. Cóclea left, the basal region bottom of a dog. Colouring with hematoxylin and eosin, 100x. LE = Limbo spiral, L = spiral ligament. The arrow points for estriavascular.
In this way, tight junctions of the marginal cells of stria vascularis separate endolymph from perilymph, which seems to free circulate in the spiral ligament on the lateral wall of cochlea. Tight junctions between cells of Reissner membrane also separate the two mentioned fluids. And, eventually, the tight junctions from the apical surface of the sensorial and supporting cells form a second barrier, preventing diffusion of the perilymph to the apical surface of hair cells, as the perilymph circulates frelly through basilar membrane. Therefore, the body of sensory and supporting cells is submerged by perilymph, while apical surface is in direct contact with endolymph. Other types of intercellular contact are present in the Corti´s organ, developing its job not only when separating the compartiment of endolymph, but aso when performing maintenance of this organ integrity during the process of dissipation of mechanical energy on sound stimulation. So, adherens junctions such as desmosome, and gap junctions are other areas of present intercellular adhesion.
In humans, cochlea presents two complet turns and three quarters of a new turn. In rodents and some mammalians such as cats and dogs, the cochlea canal is larger. Some areas inside cochlea canal are highly different and form, from epithelial cells, sensory area of cochlea - Corti´s organ - composed by external hair cells, internal hair cells and supporting cells, which rest on basilar membrane (Picture 3). Through light microscopy, it is possible to identify, in hair cells, an apical surface, where stereocilia are, and also a basolateral area, where nerve fibers get together. Such nerve fibers cross spiral lamina through the Habenulae perforatae.
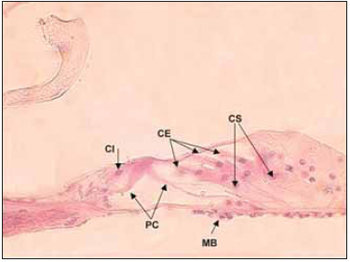
Picture 3. Fotomicrografia the organ of Corti in a section 20µ. Cóclea left, the lower basement of a dog. Colouring with hematoxylin and eosin, 400x. CI Cell ciliada internal, PC = cells of the pillar of Corti, CE = ciliated cells External, CS = Cells of support, MB = basilar membrane.
Cochlea structures can be described as spiral or radial manner. When elements are described in spiral manner, Corti´s organ rolls along basilar membrane from the base to vertex of cochlea, which is composed by around 20,000 hair cells, mixed by supporting cells. When described in radial manner, sensory epithelium is composed by internal hair cells forming a single row, in the midlle next to modiolus. External hair cells form three rows beside the internal one and they are separated from the single row by the Corti's organ pillar cells (Picute 3). Spiral and radial structures also comprise modiolar spiral and radial cochlea vessels and and other nerves.
Although the diameter of bone labyrinth reduces from the base to the cochlea vertex, membrane labytinth structures clearly increases(2). Though, in the Corti´s organ, cells in the apical turn are larger than the ones in the basal turn. The stereocilia(3) and basilar membrane are longer, while tectorial membrane is larger1 in the cochlea vertex. Such measures can vary and are related to a perceptive capacity of some frequencies. In some specimens, a variation occurs along cochlea length and in others there is an exaggeration in some cochlea postion, which agrees with functional specialization of these areas(1).
Hair cells of Corti´s organ are different in two types depending on the arrangement of their stereocilia and its ultra-structure: external and internal hair cells. In the past they were believed to be different in number and shape, but developed similar sensory functions (Picture 4). The studies of ultrastructures from hair and supporting(2,4,5) cells, as well as its innervation clarify that these cells present expressive functional differences. While internal hair cells develop sensory function in first instance, based on the fact that most afferent nerve fibers present synapses with them, the external ones change the mechnical properties of basilar membrane and of the Corti´s organ even leading stimuli of the central nercous system (CNS).
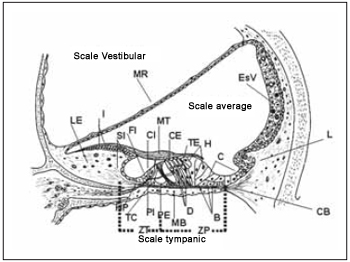
Picture 4. Schematic diagram of the average scale demonstrating its main structures and their relations with the scales vestibular and tympanic. MR = membrane of Reissner, LE = Limbo spiral, I = interdentadas cells, SI = Cells of the internal groove, FI = Cell falangeal internal, CI = Cell ciliada interaction, MT = membrane Tectorial, CE = Cell ciliada foreign, external TE = Tunnel, H = Cells Hensen, C - cells of Claudius, ESV = Estria vascular, L = ligament spiral, CB = Christian block, HP = Harbenulae Perfuratae, TC = Tunnel of Internal Corti, PI Cell internal pillar, PE = Cell Foreign pillar, MB = basilar membrane, D = Cells Deiters, B = Cells Böettcher, ZT = Zone arched the membrane Basilar, ZP = Zone pectinada the basilar membrane.
Internal hair cells are spherical-shaped with a centered nucleus and form a single row along sensory spiral epithelium. Their stereocilia are disposed in U form in apical surface and there is a filamentous bridge that links longer stereocilia to tectorial membrane. On the surface, they present microvilli aspect responsible for ionic transport with endopymph and adhesion to tectorial membrane (Picture 4). Its cytoplasm is filled by several rounded mitochondrias, Golgi complex, vesicles, lysosome and well developed endoplasmatic reticle. There is a simple layer of discontinued membranous cistern surrounding cytoplasmatic membrane. Base of cell is not in contact with basilar membrane.
Cells of internal groove or internal marginal cells form fine layers on modiolar face of internal hair cells. Internal phalangeal cells are between internal hair cells and internal pillar ones and are placed in a fine cell layer (Picture 4). Internal hair cells, similarly to the external ones, present thight junctions on reticular zone where they are in contact with adjacent cells. Therefore, differently from internal hair cells, there is no clear intercellular separation with the possibility of straight communication between adjacent hair cells next to nucleus area.
External hair cells are cylindric-like and its nuclei are in the basal portion of the cell, their diameter is smaller than the internal ones, and they occur 4 times more. In their apical surface, they are interconnected to the supporting cells, as well as in the basal surface. Therefore, in several species, their lateral surface is in contact only with fluid. Their stereocilia are disposed in W form in three or four rows, and are larger and finer than their equivalents in the internal hair cells. Cytoplasm is filled with ribosome, mitochondria and endoplasmatic reticle above the nucleus. Below the nucleus, microfilaments and microtubules are in large number and, in cellular base, there are afferent and efferent sinapses. The evidences point to the modifier role of CNS, through neuro effectors, over mechanical properties from external hair cells and then from basilar membrane. This therory is supported by the presence of receptors of Gamma-aminobutyric acid (GABA) and acetylcholine in the base of theses cells(7,8). Laterally, below cuticular plate, there is a system of membrane disposed in paralel manner, named cisterns, which are connected to cytoplasmatic membrane by structures similar to pillars. Pillars are connected by a filament chain. It is believed that this complex formed by pillars and filaments stabilize celluar surface and hold elastic and movable components that might be involved on alterations of the form of hair cells caused by sound stimulus.
Supporting cells are divided in cells with or without filaments. Externals, internal pillar and Deiters cells, have similar structure, presenting microfilaments and cytoplasmatic microtubules, forming a chain of firm support on Corti´s organ(9) (Picture 4). As mentioned before, external hair cells do not have contact with basilar membrane, and though, are subject to a expresive mechanical stress during sound stimulus. Supporting cells are specialized in the function of absortion of mechanical stress, integrity maintenance of reticular lamina, transmission of mechanical stimulus of basilar membrane to reticular lamina and of external hair cells to basilar membrane.
Internal and external pillar cells sepate internal hair cells of the external ones, forming a triangular support base to the sensory epithelium besides a tunnel filled with fluid - Corti´s tunnel, which allows the passage of nervous threads in direction to external hair cells (Picture 4). Internal pillar cells separate internal hair cells of the fluid from the Corit´s tunnel and their proportion are 1:1. In the apical surface, there are tight junctions with internal hair, external pillar and with internal phalangeal cells. External pillar cells are in larger number and the propotion is of one for each exernal hair cell. In apical surface, there are tight junctions with internal pillar, internal and external hair and Deiters cells. Some evidences show that besides its fucntion of supporting, pillar cells also transform substances by pinocytosis(10) (cell-drinking).
Deiters cells extend from basilar membrane up to the reticular lamina, and are in contact with the external hair cells. Its base is in contact with basilar membrane and its average proportion is related to the external hair cells, where there is more concentration of mitochondria and endoplasmatic reticle, which is suggested being a transport zone11. In this area, there are microfilaments that explain its supporting function. From this area, phalangeal processes go to reticular lamina. Deiters cells are enlarged from the base to the cochlea vertex and from the first row to the lateral area. Inside the cochlea there is a longitudinal gradient of organelle concentration revealing different functions in specific frequencies areas. Findings from the last decade showing the presence of creatine kinase, suggest that there is a high energetic consumption inside these cells and, therefore, other functions can be performed besides a simple supporting role11.
It is quite unknown about supporting cells that do not present organized filament structure as the previous ones. It is believed that its function is ionic transport, as they present prominent microvilli. Based on these cytological findings, there is a hypothesis that these cells take part on fluid formation that surrounds hair cells. So, Hensen cells are near the third row of Deiters cells, where they bound external Corti´s tunnel with Deiters cells of the third row. These cells rest on Boettcher cells and present a column aspect. Claudius cells are cubic-like and are placed on basilar membrane filling up the distance between Deiters cells and lateral wall of cochlea. They are not considered part of Corti´s organ, although they extend from it in lateral direction. They are different from Hensen cells for being more flattened.
Boettcher cells are found on cochlea basal turn between Claudius cells and basilar membrane, therefore their apical surfaces never are in contact with endolymph. This cellular type presents cytoplasm with more intensity than other cells on basilar membrane. Finally, cells of external groove are placed on basilar membrane junction with cells of lateral wall. Some of them are covered by Claudius cells while others are in contact with endolymph. Many others are extended in finger form towards spiral ligament next to spiral prominence (Picture 4).
Tectorial membrane (Picture 4) is a type of structure similar to gel composed by an extracellular matrix that recovers reticular lamina of the Corti´s organ and seems to develop an essential role as sensory transducer due to its close contact with stereocilia. According to its original structure description, it is similar to a dense tangle of fibers soaked in a microfibrillar matrix(4,12) There are three types of collagen and several glycoproteins in its molecular composition. It is in spiral limb and is associated with interdental cells (Ppicture 4), which are responsible for material secretion that is part of tectorial membrane composition. The three rows of external hair cells present their stereocilia emerged in tectorial membrane in all species, at least so far studied, while this association is still questioned to other internal hair cells. Tectorial membrane presents an increase from the base ti the cochlea vertex.
Basilar membrane (Picture 4) is a kind of structure over which Corti´s organ rests. It is composed especially by an extracellular matrix with fiber soaked in a homogenous substance(13). In molecular terms, it is composed by two type of collagen: II and IX(14) besides fibronectin. On tympanic scale edge, there is a layer of mesothelial cells linked among themselves by desmosomes that allows passage of perilymph. This being the case, basilar membrane is considered permeable to perilymph. Its properties change along cochlea. On the base, it is short and thick and on the vertex is wide and lean, reducing its elasticity towards cochlea vertex. Two areas are described: zona tecta or arcuate zone, which extend from the spiral limb up to external pillar cells, and zona pectinata, that extends from there up to basilar crest of spiral ligament (Picture 4). Spiral limb, basilar membrane and spiral ligament work as a functional unit on tension control.
Reissner membrane is composed by a layer of epithelial cells and other mesothelial ones that separate medium scale from vestibular scale1(6) (Picture 4). The layer of epithelial cells is aimed at endolymphatic surface while mesothelial cells are aimed to vestibular scale. Mesothelial cells are flattened and form a interrupted layer by pores extending with cells that limit vestibular scale. Epithelial cells are connected by tight junctions and are separated from mesothelial cells by a basal membrane. Reissner membrane presents high metabolic activity despite being poorly vascularized.
Information on cochlea innervation is from former studies when it was discovered that some nerve fibers go from the cochlea towards CNS - afferent fibers - and others, from CNS to periphery - efferent fibers(17,18). After these studies, other findings were reported on innervation standard and synapses structure on hair cells. Regarding structures, two findings perform expressive roles in one afferent synapsis on hair cells: the thickening of pre and post-sinaptic membranes and a pre-sinaptic terminal with vesicles on hair cells. Yet, efferent synapses with hair cells present an accumulation of vesicles on nerve terminal and a subsynaptic cistern on hair cells. Some differences were checked according to the studied species.
In general, cochlea innervation can be described as it is performed by two systems(19). Regarding afferent system, studies show that using different techniques, it is divided into two types of fibers. Myelinized thick fibers that arise from ganglion cells type I (I) and are connected to internal hair cells, and non-myelinized fine fibers that arise from glanglion cells type II (II) and are connected to external hair cells. These two groups can be divided into other five ones. Efferent innervation is divided into two subsystems. Medial system, which performs synapses with external hair cells, and lateral system, that is connected to afferent fibers from internal hair cells (Picture 5).
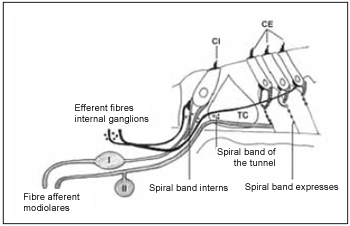
Picture 5. Schematic diagram of the two systems innervation the cochlea - afferent system (gray) and efferent (black). The afferent system is composed of two types of fibers. Fibre afferent of type I connected to the type of ganglion cells I (I) are myelinated. The afferent fibers of type II connected ganglion cells of the type II (II) does not have the sheath of myelin. These fibers inervam the ciliated cells internal (CI) and external (CE). The system efferent crosses the tunnel of Corti (TC) inervar ciliated cells to the external (CE). Cells internal also are innervated by the efferent system.
First studies on cats(6) show that around 90-95% of fibers that are on acoustic nerve perform synapsis with internal hair cells and each cell receives around 20 nerve fibers(23). The number of fibers that inervates each hair cell ranges inside the cochlea and do not follow a gradient. This number seems to be greater in important areas in terms of function. Though, this number, in cats and in humans, is greater on hair cells in the middle of cochlea. According to morphological and topographic features, these fibers can be divided into two types: 1. fibers with large diameter, low threshold of excitation and with a high rate of spontaneous despolarization that perform synapsis next to pillars; 2. fibers with smaller diameter, high threshold of excitation and low rate of spontaneous despolarization that are placed next to modiolus. All fibers go from spiral bone lamina to an area on tympanic edge of basilar membrane named habenulae perforate. From this point, they become myelinized. Ganglion cells type I are in larger number and comprise around 95% of all cells of spiral ganglion. They extend in central and peripheral manner. Its cellular body is rich in mitochondria, endoplasmatic reticle, Golgi complex, ribosome, microtubules and neurofilaments.
Efferent fiber system of the internal hair cells arises from CNS, in the superior and lateral proportion of ipsilateral olivary and contralateral complex on brainstem, then penetrates cochlea on basal and middle area and go to the base and vertex of cochlea. These fibers, in a radial manner, go through cells of spiral glanglion and penetrated spiral bone lamina by habenulae perforatae when change into non-myelinized fibers. These fibers that correspond from 50-100% of efferent fibers that penetrate the cochlea are divided inside bone lamina and form varicosities before performing synapses with dendrites of the afferent system that leave the internal hair cells. Areas of direct synapses with internal hair cells(24) that occur especially in young animals(25) were described. Areas with mutual synapses, where the same nerve presents both afferent and efferent synapses, were found on internal hair cells in cultures of Corti´s organ(26).
Afferent innervation of external hair cells is highly divided, in a way that each cell performs synapsis with afferent fibers(27). In this way, each nerve fiber can receive information from 6 to 100 external hair cells, especially from the same cochlea turn. Standard division of afferent fibers is not uniform and change systematically from base to cochlea vertex. In general terms, density of afferent innervation from external hair co-relates with the number of non-myelinized fibers that cross the base of spiral bone lamina and with the number of ganglion cells type II that provide the area. This being the case, the larger number of ganglion cells type II connects to external hair cells of the first cochlea turn. Most of the other ganglion cells connect to external hair cells of the cochlea vertex.
In terms of structure, synapsis with afferent fiber presents a depression on hair cell and evagination on the surface of nerve terminal. There is a gap between both terminal from where vesicles and electrondense material can be seen. Different from afferent fibers that connect to internal hair cells, afferent fibers of external hair cells do not present myelin layer when emerging from habenulae perforatae. After following radial route, such fibers connect with cellular body of ganglion cells typr II. Such cells are smaller than cells type I and are pseudo-unipollar ones. They do not present myelin layer and are involved by a layer of Schwann cells.
Efferent innervation system of the external hair cells arises from fiber that leaves from cellular body of the medial superior olivary nucleus. Projections can be ipsilateral and contralateral, which go towards to cochlea edge through spiral intra-ganglion area. After penetrating through habenulae perforatae, fibers remain myelinized¸spiral inside bone cells, cross Corti´s tunnel and, in radial direction, go towards external hair cells28. Being next to hair cells, those ones divide in all direction and ends with a dilatation full of vesicles on the base of external hair cells. Some efferent fiber ends on the afferent system in a junction axodendritic-like(29).
Some studies have showed that there are important similarities between vascular anatomies of the cochlea on different species of mamalians including humnas(30). The general arrangement of vessels present a spiral component - modiolar artery and modiolar vein - and a radial component - radial arteriole and collector vein. In this way, calibrous arteries and veins spiral on modiolus, while smaller vessels present a radial course on spiral bone lamina in order to supply cells and lateral wall of cochlea (Picture 6).
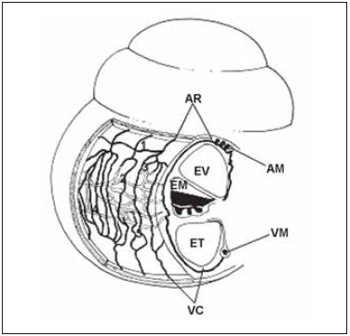
Picture 6. Schematic diagram of cochlear vascularization. (AR), radial arterioles, which is the branch artery modiolar spiral (AM) and follow a course by the side wall bone above the scale vestibular (EV). Its branches serve the capillaries above-estriais (1), which form the capillary network capillary string of vascular (2), the arcades arteriovenous (3), capillaries of the spiral prominence and capillaries in the region bottom of the spiral ligament (5). The capillaries are drained by collecting venules (VR) which leave the side wall of cochlea by bone below the scale tympanic (ET) draining modiolar spiral into the vein (VM). EM = scale media).
There are expressive differences in this vascular system along cochlea. Vertex presents few radial arteriole and fine stria vascularis. Yet, the base of cochlea presents thick stria vascularis with radial arteriole well organized that follows its course along inferior edge of stria vascularis. In this area, there is a greater number of communications between arteries and veins on spiral ligament that deviates blood flow from stria vascularis. It is essential that capillary vessels on lateral wall of cochlea present a wide diameter and often are seen with erythrocytes in its interior. In cochlea from old animals or with cochlea disease, it is possible to find avascular or capillary areas with minimum light, and many times with no erythrocytes in its interior.
Artery and spiral modiolar vein are predominant vascular structures and follow a winding course along modiolus. Radial arterioles are branches from modiolar artery that follow to lateral wall of the cochlea through the bone above vestibular scale. The branches from these arterioles supply capillary vessels from suprastrial area (1) and from stria vascularis (2), venous artery arch (3), capillary vessels from spiral prominence (4) and inferior capillary vessels of spiral ligament (5). Cappilary vesses are drained by collector veins that leave lateral wall of the cohclea by the bone that recover tympanic scale draining on spiral modiolar vein (Picture 6).
There are three areas of cochlea that present cells and similar vascularization in structure and that are probably related with ion transport. The area of spiral ligament above stria vascularis, spiral prominence and limb area, near insertion of Reissner membrane are highly vascularized and fibroblasts with cytoplasm rich in ribosome, endoplasmatic reticle, Golgi complex and mitochondria. Such cells do not present tight junctions and there is high Na+-K+-ATPase activity(31). The presence of these similar fibroblasts in both sides of Reissner membrane suggest that they should be involved with production and/or maintenance of perilymph or even regulate electrolytic balance between blood and perilymph.
Spiral ligament is composed by conjunctive tissue, epithelial cells, blood vessels and an extracellular matrix. It is placed next to optical capsule and provides the support to stria vascularis and to basilar membrane, to which it is connected. In the superior and inferior area, fibroblasts are in straight contact with perilymph of the vestibular and tympanic scales. Farther stria vascularis, spiral ligament is in contact with cells of basal layer. In the inferior area, i.e., spiral prominence, epithelial cells connected by tight junctions prevent blending of perilymph from tympanic scale with endolymph from medium scale. According to Henson(32), spiral ligament can be divided into five area in conformity with orientation, form and relation of conjunctive tissue with extracellular matrix. These areas are kept in several species. Peripheral area is behind stria vascularis. Central area is in spiral prominence area. Subcentral area is near to basilar crest, next to insertion of basilar membrane. Hyaline area is inside basilar crest and next to subcentral area. Marginal area is composed by fibroblasts related with tension of basilar membrane. Cells of external groove, next to spiral prominence, are cubic-like whose apical surface is in contact with endolymph. In some species, such cells are covered by Claudius cells. They present high Na+-K+-ATPase activity as well as cells of spiral prominence and therefore, they should develop function on regulation of fluid from cochlea canal (Picture 7).
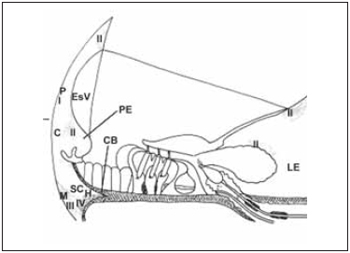
Picture 7. Diagram of a section through the stria vascularis (EsV) and the spiral ligament illustrating the areas of basic spiral ligament and the types of fibroblast according to the enzyme content. The region perififérica (P) is located behind the stria vascularis (EsV). The central region (c) includes the apical process cells from outside groove, near the spiral prominence (EP). The region subcentral (SC) is located below the central region (C). The region Marginal contains fibroblasts responsible for anchoring the spiral ligament to bone side wall of cochlea (CO). A hyaline region (H) is located within the crest basilar (CB). The fibroblasts of type I (I) contain carbonic anhydrase and creatine kinase. The fibroblast of type II contain ATPase K + and Na +. The fibroblast type III (III) contain anhydrase carbonic and creatine kinase. The fibroblasts of type IV (IV) enzyme content are variable.
According to division of spiral ligament proposed by Henson(32), cellular stroma can be classified in four types of fibrosblasts that roughly follow the areas proposed by Henson. Fibroblasts type I (I) holds a large amount of Creatine kinase and Carbonic Anhydrase. They are homogeneously distributed in posterior area at stria vascularis. Fibroblast type II (II) is placed in supra-stria area, above limb and in the area of spiral prominence. Their cytoplasm is rich in Na+-K+-ATPase and mitochondria. Fibroblasts type III (III) are next to optical capsule, in the inferior portion of spiral ligament and present cytoplasm with carbonic anhydrase and Creatine kinase. Fibroblasts type IV (IV) are placed more superficially in a inferior portion of spiral ligament and its cytoplasm holds carbonic anhydrase and Na+-K+-ATPase. Besides the presence of Na+-K+-ATPase enzyme in large quantity, morphological observations confirm that fibroblasts are involved with ion transport. Fibroblasts type II are connected among themselves and to cells of basal layer of stria vascularis by many gap junctions(33), what affects electrical or ion association between them. Potassium is believed to be pumped out of perilymph, spreading among cells of spiral ligament up to stria vascularis, and then pumped towards endoplymph. Therefore, to some authors34, high concentration of fibroblasts type II on basal area of cochlea can be responsible for generation and maintenance of large endocochlea potential evaluated in this area (Picture 7).
Stria vascularis is composed by highly vascularized epithelial tissue and is placed in the lateral wall of cochlea. It is considered the only epithelial tissue that presents vessels placed between two layers of cells joined by tight junctions. Cells are rich in Na+-K+-ATPase(31). It is believed, therefore, that stria vascularis segregates potassium in medium scale, contributing directly to genesis of endocochlear potential. Once stria vascularis perform an important role on cochlea function, several morphometric studies were done in order to evaluate its length, width, relative volume of cell as well as capillary vessels and number of marginal cells(35,36,37). There are basically three types of cells in stria vascularis: marginal, intermediate and basal ones. Interstitial space is protected from endolymph by tight junctions of cells of marginal layer(38), as well as from diffusion of perilymph by the presence of tight junctions of cells of basal layer(38). Nevertheless, there are gap junctions that join cells of basal layer to adjacent fibroblasts from spiral ligament and to cells of intermediate layer(39). Therefore, glucose and potassium can be taken in active manner from perilymph through cell of basal layer to cells of marginal layer. This selective access of ions and metabolites to perilymphatic space associated to limited permeability of capillary vessels of stria(40) reinforce the hypothesis that endolymph should be from perilymph and not from blood.
Cells of marginal layer are darkish with some microvilli on its apical surface directed to endoplymph. Their cytoplasm is rich in mitochondria, microtubules and cytokeratin. There is a linear relation between the size of stria vascularis the number of cells of marginal layer. Cytoplasmatic extensions of cells of marginal layer surround most capillary vessels inside stria vascularis. Cells of this layer are joined among themselves by tight junctions and adherens junctions. Below junction complex, the basolateral membrane of each cell doubles and its projections are associated to mitochondrias. This being the case, lateral surface of each cell presents specialized interdigitation and extracellular space is scarce. These cells present ATPase Ca2+ activity in apical membrane(41) and Na+ K+ ATPase in their basolateral membranes31 besides being colored positively to creatine kinase(42,43). Finally, some studies show that in the interior of cytoplasm of marginal cells there is a well developed endoplasmatic reticle, high concentration of vesicles, vacuoles and glycogen(42). These specializations are cells features with metabolic activity with secretory transport functions(44).
Intermediate cells form an interrupted layer along marginal cells. They are in contact with endolymph and form a group of clear cells. The fact of being in intermediate position allows them to be in contact with cells from basal and marginal layer, and then it is explored that they perform an important role when generating positive component of endocochlear potential. Despite being considered 'clear' cells of stria vascularis, it is believed that these cells are melanocytes from neural crest with capacity of melanin production.
Conlee and col.(45), working on albinic guinea pigs, whose melanocytes are amelanotic, i.e., they do not synthesize pigment. They also verified that cells of intermediate layer occupied less room than usual and that there was a compensating increase on volume of cells of marginal layer. Mutating guines pigs with white spots presented absence of intermediate layer cells and endocochlear potential was nearly zero. This suggests that intermediate cell activity is essential to the development or function of marginal layer cells or to advance potassium movement towards endolumph(46).
Basal cells are flattened and joined by tight junctions forming a continuous layer of cells that separates the other cells and capillary vessels from stria vascularis of spiral ligament and from perilymph. This layer extends above and below stria vascularis and is in contact with endolymphatic surface. Tight junctions placed between basal cells on endolymphatic surface are more fused than those from other areas of cochlea. There are gap junctions connecting basal cells among themselves, joining basal and intermediate cells and, finally, joining basal cells to cells of spiral ligament. In Gerbils, the protein transporter of GLUT1 glucose seems to be a particular feature of basal layer cells of stria vasularis(44). It is believed, therefore, that glucose is transported from interstitial space of spiral ligament by basal cells of stria vascularis.
Vestibular system is composed by vestibule, where saccule and utricle are placed and by semicircular canal. Similar to cochlea, it is divided into a bone part and membrane portion. Saccule is placed on spherical recess of vestibule. In humans and on most mammalians, it is in vertical position when cephalic pole of the animal is in orthostatic position. Its membranous wall is in contact with utricle in superior manner. Lateral portion of membranous wall presents a thickening - reinforced area - and is placed next to bone wall of vestibule. Sensory epithelial of the saccule, named macula, is in vertical position. Saccule communicates with endolymphatic canal by saccular duct and with cochlea by reunens duct. Utricle consists of an oval and irregular tube that is placed on epithelial recess of superior vestibule at saccule. Utricular macula is placed on horizontal plan. Endolymphatic canal communicates with endolymphatic sac.
The three semicircular canals communicate with utricle by five openings, one of those is formed by junction of non-ampular extremity of the superior and inferior semicircular canals, named common cross. Next to utricle, each canal presents dilatation on its extremity named ampulla. A type of septum similar to a crest crosses ampule base and suffers a differentiation forming sensory epithelium, which is distributed over conjunctive tissue, blood vessels and nerve fibers. Sensory organ of the ampulla is covered by a gelatinous cupola that extends to the other extremity of the crest wall. At the end of each crest, there is an amount of cylindriclike cells forming a half moon shape named semilunar plane. In this transitional area, there is an accumulation of dark cells that develops a secretory function.
Vestibular sensory epithelium is in the macula of saccule and utricle and in the crests of semicircular canal. Its structure is relatively preserved between different species of mamalians. This area, being similar to cochlea, holds two types of hair cells with different functions(46). Hair cells type I, represent internal hair cells of the cochlea. They are shaped like ancient Greek wine bottles and are surrounded by a nerve chalice. Casually, two or three hair cells are joined by the same nerve chalice. Hair cells type II, which are similar to external hair cells of cochlea, are shaped like cylinder. In phylogenetic terms, hair cells type II can be found in inferior vertebrate and are considered older than cells type I. Hair cells type I are in larger number in he ampullary crest, while in macula they are in the same number as the cells type II. Phylogenetically, hair cells type II arise in the vertebrate inferior animals and are considered more ancient than cells type I. Hair cells type I have prevalence in ampular crests, while in the maculas, they are in the same number as cell type II.
Cilium can present different sizes in different vestibular sensory organs. They are smaller and in smaller number in the maculas, and are soaked in otolith cupola. There seems to be a not well clarified anatomical and functional polarity in the interior of macula and crests. In humans, there seems to be another sensory area, and inconsistent, named neglecta crest, next to ampular crest of posterior semicircular canal. In some mammalians, such as cats, this amount remains individually. Afferent innervation is represented by myelinized fibers that are more thickened when related to hair cells type I. Fibers that innervate crests also present a thicker myelin surrounding. Efferent fibers are in straight contact only with hair cells type II. Several cilia project from the free edge of hair cells. Hair cells from crests present greater innervation than the hair cells from the macula(47,48). The velocity of conduction inside nerve fiber is proportional to the diameter of the fiber. By the features already described, perception of angular acceleration, captured by crests, occurs quicker that perception of linear acceleration, which is captured by maculas.
Otolith membrane and cupule recover sensory organs of macula and crist respectively. Otolith membrane is composed by a gelatinous layer, formed by fibers where crystals of otoconial layer rest. Subcupular layer supports gelatinous layer and rest on sensory cells. In humans, otoconial layer is composed by crystals of sodium carbonate and small quantities of sodium, magnesium, phosphate, chlorine and potassium. Larger crystals rest on hair cells. It is believed that these substances are segregate by supporting cells of the macular sensory epithelium. Otoconial layer maturation occurs in late gestation period, when it is possible to verify crystals in an immature form in newborns. Otoconial layer seems to be particular of the saccule and utricle, therefore, deteriorated crystals can be seen next to the surface of dark cells in the cúpula of posterior semicircular canal. This is believed due to detachment of the changed utricle otoconial layer(49).
Primary vestibular neurons, which are inside Scarpa ganglion, in lateral part of the internal hearing canal, are bipolar cells whose peripheral extensions are towards vestibular sensory and central organs, towards vestibular nuclei and cerebellum. Vestibular nerve is divided into two trunks. Superior vestibular branch follows facial nerve track, which is superior to transverse crest - a bone projection on internal hearing canal - and is dived into two other branches in order to supply lateral and anterior ampular crest, besides utricle. Inferior division follows cochlea nerve below transverse crest and is divided to innervate posterior ampulla and saccule. Scarpa ganglion is also divided by transverse crest into two parts in humans.
Despite cochlea maturation occurs during the twenty-fifth week of gestation in humans and during post-natal life in other mammalian, vestibular neural elements develop more slowly and after cochlea maturation. In the same way, loss of these elements is early, and in humans, such loss occurs around 60% at the age of eighty.
Endolymphatic sac follows in partial manner in the interior of a bone area in posterior surface of petrous bone and inside durameter of posterior fossa. It is connected to the endolymphatic system by endolymphatic canal, which begins in vestibular aqueduct - a bone canal in the vestibule. Its light is extremely irregular, with profound folds filled by cellular rests and structures similar to otoconia. Proximal area presents epithelial covering similar to endolymphatic canal. Intermediate area, which is more distinct, is covered by high cylindric epithelium with many papilla and crypts. Distal portion is associated to durameter.
Finally, cochlea aqueduct, a small tube filled by fluid, connects perilymphatic space of tympanic scale to cerebrospinal fluid of subarachnoid space. It is formed by a bone canal. Its function seems to be related with maintenance of pressure and fluid between inner ear and subarachnoid space. It was first described in 1684 by Du Verney and systematically measured by Bachor in 1997 through a histological study of the temporal bone in children(50, 51).
FINAL CONSIDERATIONSKnowing basic areas that compose medicine is essential to formulate proper clinical argument. In otology area, there are a large number of injuries in inner ear resulting few signs and symptoms that are not enough to establish a final diagnosis. Therefore, understanding pathology is the base for prevention and treatment founded on evidences of such diseases. However, such base would not be possible to be reached without late advances that would help in clarifying structures and ultra-structures of the inner ear.
This current revision was based on original articles by authors who contributed, in a crucial way, in helping to understand inner ear functioning after establishing several of its compartments. Doing systematic revision of scientific literature is often full of imperfections due to a large number of information gathered in geometrical proportion. References of this work are based on original studies. On the other hand, it is impossible to mention all the others who added some information. We would like to thank the authors previously mentioned for being helpful in order to establish a solid base when studying inner ear.
CONCLUSIONSAnatomical studies of the cellular compartment of inner ear from mammalians allow understanding different functions that each of them performs in sound perception. Therefore, cells do not work separately, and hearing depends on interactions among these cell gatherings. Some interactions occur between cells related in structure manner and other cells separated in space manner. Most of recent researches focus on nature of these interactions.
Some examples of questions are: the relation between stereocilia of hair cells and tectorial membrane and modification of this interaction with age and after acoustic trauma, and the way how cells hold their position inside sensory epithelium of cochlea and the way of renovation of these cells. Finally, the study of structure and ultra-structure of inner ear will be able to clarify basic questions on the relation between damage to lateral wall of cochlea - stria vasularis - and reaction on hearing, or how morphological alterations are correlated with physiological changes of nerve fiber that connect to hearing system peripheral way.
REFERENCES1. Echteler SM, Fay RR, Popper AN. Structure of the mammalian cochlea. In: Fay RR, Popper AN (editors). Comparating hearing: mammals. 1st ed. New York: Springer Verlag; 1994. p.134-171.
2. Lim DJ. Functional structure of the organ of Corti: a review. Hear Res 1986;22:117-146.
3. Roth B, Bruns V. Postnatal development of the rat organ of Corti. Anat Embryol 1992;185:571-581.
4. Kimura RS. Hairs of the cochlear sensory cells and their attachment to the rectorial membrane. Acta Otolaryngol 1966;61:55-72.
5. Angelborg CA, Engström H. The normal organ of Corti. In: Moler AR (editor). Basic Mechanisms in Hearing. 1st ed. New York: Academic Press; 1973. p.125-182.
6. Spoendlin H. Innervation of the cochlear receptor. In: Moller A (editor). Basic Mechanisms in Hearing. 1st ed. New York: Academic Press; 1973. p.185-230.
7. Zenner HP, Reuter G, Plinkert PK, Zimmermann U, Gitter AH. Outer hair cells possess acetylcholine receptors and produce motile responses in the organ of Corti. In: Wilson JP, Kemp DT (editors) Cochlear Mechanisms. 1st ed. New York: Plenum Publishing Corp; 1989. p.93-98.
8. Plinkert PK, Gitter AH, Zimmerman U, Kirchner T, Tzartos S, Zenner HP. Visualization and functional testing of acetylcholine receptor-like molecules in cochlear outer hair cells. Hear Res 1990;44:25-34.
9. Angelborg C, Engström H. Supporting elements in the organ of Corti. Fibrillar structures in the supporting cells of the organ of Corti of marnmals. Acta Otolaryngol 1972;301(1 Suppl):49-60.
10. Preston RE, Wright CO Pinocytosis in the pillar cells of the organ of Corti. Acta Otolaryngol 1974;78:333-340.
11. Spicer SS, Schulte BA. Cytologic structures unique to Deiters cells of the cochlea. Anat Rec 1993;237:421-430.
12. Lim DJ. Fine morphology of the rectorial membrane. Arch Otolaryngol 1972;96:199-215.
13. Angelborg C, Engström B. The tympanic covering layer. An electron microscopic study in the guinea pig. Acta Otolaryngol. 1974;319(1 Suppl):43-56.
14. Halmann L. Collagen of accessory structures of organ of Corti. Conn Tiss Res 1993;29:199-201.
15. Keithley EM, Ryan AF, Woolf-NK. Immunoreactivity of the basilar membrane of young and aged rats. J Comp Neurol. 1993 327:612-617
16. Duvall AJ, Rhodes VT. Reissner's membrane: an ultrastructural study. Arch Otolaryngol 1967;80:143-151.
17. Rasmussen G. Studies of the Vlllth cranial nerve of man. Laryngoscope 1940;50:67-83.
18. Engström H. Structure an innervation of the inner ear sensory epithelia. Int Rev Cytol 1958;7:535-585.
19. Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Ver 1993;73:309-373.
20. Kimura RS. An electron microscopic study of cochlear nerve fibers followed serially from spiral ganglion to organ of Corti. Ear Res Jpn 1986;17:4-7.
21. Ginzberg RD, Morest DK. A study of cochlear innervation in the young cat with the Golgi method. Hear Res 1983;10:227-246.
22. Romand R, Hafidi A, Despres G. Immunocytochemical localization of neurofilament protein subunits in the spiral ganglion of the adult rat. Brain Res 1988;462:167-173.
23. Spoendlin H, Schrott A. The spiral ganglion and the innervation of the human organ of Corti. Acta Otolaryngol 1988;105:403-410.
24. Kimura RS. The ultrastructure of the organ of Corti. lnt Rev Cytol 1975;42:173-222.
25. Emmerling MR, Sobkowicz HM, Levenick CV, Scott GL, Slapnick SM, Rose JE. Biochemical and morphological differentiation of acetylcholinesterase - positive efferent fibers in the mouse cochlea. J Elect Microscop Tech 1990;15:123-143.
26. Sobkowiez HM, Slapnick SM, August BK. Presynaptic fibers of spiral neurons and reciprocal synapses in the organ of Corti in culture. 3 Neurocytol 1993;22:979-993.
27. Dannhof BJ, Bruns V. The innervation of the organ of Corti in the rat. Hear Res 1993;66:8-22.
28. Spoendlin H. Neural connections of the outer hair cell system. Acta Otolaryngol 1979;87:381-387.
29. Guinan JJ, Warr WB, Norris BE. Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J Comp Neurol 1982;221:358-370.
30. Axeisson A, Ryan A.Comparative study of the vascular anatomy in the mammalian cochlea. In: Jahn AF, Santos-Sacchi JR (editors). Physiology of the Ear. 1st ed. New York: Raven Press; 1988. p.295-316.
31. Schulte BA, Adams JC. Distribution of immunoreactive Na', K'-ATPase in gerbil cochlea. J Histochem Cytochem 1989;37:127-134.
32. Henson MM, Henson OW. Tension fibroblasts and the connective tissue matrix of the spiral ligament. Hear Res 1988;35:237-258.
33. Takahashi T, Kimura RS. The ultrastructure of the spiral ligament in the rhesus monkey. Acta Otolaryngol 1970;69:46-60.
34. Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res 1991;56:53-64.
35. Santi PA, Lakhani BN, Bingham C. The volume density of cells and capillaries of the normal stria vascularis. Hear Res 1983;11:7-22.
36. Lohuis PJFM, Patterson K, Rarey KE. Quantitative assessment of the rat stria vascularis. Hear Res 1990;47:95-102.
37. Carlisle L, Forge A. The vessels of the stria vascularis: quantitative comparison of three rodent species. Hear Res 1989;38:111-118.
38. Reale E, Luciano L, Franke K, Pannese E, Wermbter G, lurato S. Intercellular junctions in the vascular stria and spiral ligament. J Ultrastruct Res 1975;53:284-297.
39. Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junction systems in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol 1995;191:101-118.
40. Santos-Sacchi J, Marovitz WF. An evaluation of normal strial capillary transport using the electron-opaque tracers ferritin and iron dextran. Acta Otolaryngol 1980;89:12-26.
41. Yoshihara T, lgarashi M. Cytochemical localization of calcium ATPase activity in the lateral cochlear wall of the guinea pig. Arch Otorhinolaryngol 1985;243:395-400.
42. Spicer SS, Schulte BA. Creatine kinase in epithelium of the inner ear. J Histochem Cytochem 1992;40:185-192.
43. Conlee JW, Gerity LC, Westenberg IS, Creel DJ. Pigmentdependent differences in the stria vascularis of albino and pigmented guinea pigs and rats. Hear Res 1994;72:108-124.
44. Schulte BA, Steel KP. Expression of alfa and beta subunit isoforms of Na, K-ATPase in the mouse inner ear and changes with mutations at the Wv or SI d loci. Hear Res 1994;78:259-260.
45. Ito M, Spicer SS, Schulte BA. Immunohistochemical localization of brain type glucose transporter in mammalian inner ears: comparison of developmental and adult stages. Hear Res 1993;71:230-238.
46. Engström H, Ades HW, Hawkins JE. The vestibular sensory cells and their innervation. Symp Biol Hung 1965;5:21-41.
47. Rosenthal, U. Vestibular macular mapping in man. Ann Otol Rhinol Laryngol 1972;81:339-352.
48. Rosenthal, U. Mapping of cristae ampullares in man. Ann Otol Rhinol Laryngol 1972;81:882-891.
49. Schuknecht HF. Anatomy. In: Schuknecht HF. Pathology of the ear.1st ed. Cambridge, MA: Havard University Press; 1974. p.31-75.
50. Bachor E, Byahatti S, Karmody CS. The cochlear aqueduct in pediatric temporal bones. Eur Arch Otolaryngol 1997;132(1 Suppl), p.8-34.
51. Brenski AC, Arjmand EM. Congenital inner ear anomalies. In: Bluestone CD. Pediatric Otolaryngology.4th ed. Philadelphia: Saunders; 2003. p.441-455.
1. PhD in Health Science (Assistant Doctor at ENT Service - UnB)
2. PhD, University of Minnesota Professor - Titular Professor of ENT discipline - UnB - (Head of ENT Service at Hospital Universitário de Brasília)
Universidade de Brasília - UnB Faculdade de Medicina da Universidade de Brasília Hospital Universitário de Brasília Serviço de Otorrinolaringologia
Andre Luiz Lopes Sampaio
Address: SQN 205 Bloco B apto 506 70843020-Asa Norte Brasília - Brasil andremarjy@uol.com.br fax: 55 61 34433397
Capes - Fulbright Programa de Doutorado sanduiche no exterior
This article was submitted to SGP - Sistema de Gestão de Publicações (Publication Management System) from RAIO on July 04, 2006 and was approved on August 07, 2006 15:02:29.