INTRODUCTIONIFOS (
International Federation of Oto-Rhino-Laryngological Societies) estimates that about 10% of the worldwide population are hearing impaired people. The Better Hearing Institute states that 10% of the USA population have some kind of hearing impairment. The 2000 Brazilian Census identified 5.735.099 hearing impaired people, a number larger than that of mental or physical handicapped and slightly lower than the number of motor handicapped. Researchers of the Lutheran University in Canoas (RS), in a 2004 study, identified 6.8% of the local population with incapacitating hearing impairment. Out of these, 5.4% with moderate losses, 1.2% with severe losses and 0.2% with deep losses (1). Taking into account the current Brazilian population of about 190 million of inhabitants, we would compute more than 10 million of incapacitating hearing impairments (over 41 dB). With the 10% standard, the Brazilian hearing impaired would reach 19 million of people or the whole population of Denmark, Norway and Sweden altogether.
Many are the causes contributing for the increase of this number, among which presbyacusis, hereditary diseases, metabolic diseases, the use of ototoxic drugs, acoustic traumas, excess of noise, neoplasms, infections and vascular damages. Among the resulting effects we remark anxiety, frustration, paranoia, lack of confidence, emotional instability, depression, social phobia, sensation of frustration and incapacity for orientation (2).
In the clinical audiology point of view, every hearing impaired person is strongly prone to use a Personal Sound Amplification Device or hearing aid. With the increase of the world population longevity, we can state that deafness will be one of the twenty-first century's diseases.
The core of a digital hearing aid is its electronic configuration that comprises a computerized circuit for the processing of signs or (DSP) digital signs processor and both the input (microphone) or the output (receptor) transducers (5, 6).
The current values of international industries dominating this market prevent the access of millions of hearing impaired people whether for hearing enabling or rehabilitation. Therefore, a low-cost hearing aid designed under a generic electronic circuit could become an option instead of the imported hearing aids. It was through Decree no. 626 of March 23, 2006 providing hearing health attention services and the physical and financial limits of States, Federal District and Cities, that the Federal Government invested R$ 13.764.814,80 in the use of hearing aid by 4.903 individuals at a cost of R$ 2.807,43 per hearing impaired person. With the Government participation in these policies, the retail customers may also be benefited for there is a rational demand for the use of hearing aid.
The purpose of this work is to develop 25 low-cost digital hearing aid prototypes, specially in the BTE design or retroauricular, placed under a generic electronic circuit, that have electroacoustic resources not lower than the same design hearing aids and with the same level of those traded in the Brazilian market according to decree no. 626.
We have defined the generic electronic circuit as that allowing the assembly of several hearing aids models based on the same electronic components, which result in lower costs in the development, a better domain on the variables of production and technical assistance, lower storage costs and lower operational cost.
We have sought to develop a hearing aid that meets the hearing losses classified from moderate-severe (from 56 to 70 dB), a range that includes the highest levels of amplification. The hearing aid design set is BTE for it enables a number of benefits directly compared to the (CIC) completely-in-the-canal designs, known as micro canals or ITC (in-the-canal) called intra-canals. Among these benefits:
I - It allows for a larger area for tests with different components (has a wide box).
II - It allows for the measuring of ready products final parameters for immediate prosthetization.
III - Offers the shorter prosthetization time compared to other designs.
IV - Allows for the insertion of a greater number of possible resources in the same product.
V - In a test with patients, it allows for a direct comparison with BTEs of other trademarks because the same patient's mould is used.
VI - Meets the World Health Organization's requirements in its hearing aids supply program for developing countries (8).
VII - Allows the access for the greatest number of patients.
VIII - Offers a level of breakages lower than the CICs and ITCs.
METHODAll the development project for these hearing aids was performed at the Medical Research Laboratory of the Clinical Hospital of University of São Paulo - LIM-32.
10 phases are pointed out:
1 - Identification of components manufacturers.
1.1 - The microphones and receptors are the sound pressure transducers. We have set forth the Knowles' transducers for this supply.
1.2 - DSP (Digital Signal Processing) - the signals digital processor that is the main component. We started our project in 2006 with GA3216, but after almost two years we migrated to GA3226, both by Sound Design Technologies Ltd. (SDT), the new corporate name of Gennum Corp's DSP's division for hearing aids.
1.3 - Plastic box - a box in which the components remain. We set up the box and its components by In'Tech Industries Inc.
1.4 - Other parts.
Potentiometer, socket, programming button and telephone coil were set up like the ones by Deltek (Knowles division). The microphone and receptor suspensions and tubes were made of silicon tubes available in the Brazilian market.
2 - Components identification.
For the components selection process we use QFD (Quality Function Development) that is a Japanese technique used in the products development.
3 - Components acquisition.
Except for the silicon tubes, all other components have been imported. The import process begins with a quotation request and results in the parts delivery by an international supplier.
4 - Generic electronic circuit build-up.
4.1 - Creation of a map (definition of the electronic circuit components);
4.2 - Build up of a library (definition of all resources, adjustment intervals, operational levels setup and others) (10,11).
For the setup of the map and the library, an on-line connection is made to the SDT server through the set of ARKs (Application Resource Kits T), which demands authorization and the use of a password supplied by SDT (12, 13).
From the SDT server we then obtained the DLLs (libraries) that are downloaded from the server directly to the computer. A set of complimentary applications was installed to the computer from ARK:
I - ARK Component Manager (the basic function is to enable the install and uninstall of libraries);
II - Controller Toolbox (certifies the type of controller and verifies the electronic connectivity);
III - Interactive Data Sheet (enables the customization of the hearing aid functioning conditions and the hearing aid acknowledgment in the fitting program).
5 - Mounting of the circuit and welding of the components.
The electronic circuit mounting was made according to the model GA3226 DSP pins available in the respective manual. The welding process of all the components met the specifications of the aforesaid manufacturers (14, 15, 16).
6 - Connectivity Checking.
The circuit's operability was checked through the blasting observed when it is fed with a battery. However, the electronic connectivity is only ensured when the softwares and other features listed in item 4 are used.
7 - Features customization.
All features intrinsic to the hearing aid functioning shall be determined in this step, as well as the features to which the phonoaudiologist will have access.
8 - Stethoscope tests.
After mounted the hearing aids were submitted to a sorting, that is, fed with new batteries and previously tested with the use of stethoscope. A trained ear enables the identification of problems before going on with the tests on acoustic research equipment.
9 - Tests with acoustic research equipment.
The acoustic research equipment used was the model CAS of Danavox trademark, operated by an independent laboratory.
The mounted BTE was named Manaus.
10 - Software performance for fitting.
The fitting program is availed by DST and was customized with the input of pictures, texts etc., through the Tool Customization application, under license of SDT. The fitting was translated into Portuguese and named AdaptEASY. It works very well even in modest configurations such as Pentium 2, with 512 Mbytes of RAM and hard disk of 4 Gbytes, and doesn't demand high investments in hardware for the phonoaudiologist. The universal programmer used was HI-PRO (GN ReSound trademark). By means of a programming socket, the hearing aid is connected to the HI-PRO by a communication cable type CS44 standardized in the industry. The programming adjustments set up in the AdaptEASY are recorded in the hearing aid memory.
Described concisely the signals processing occurs from the sound pressure variations, which are captured by the microphone and initially pass by an analog-digital converter where the input electric signals (analog) are transformed into digital pulses. The programs are stored like mathematical instructions, which allows these signals' digital processing and storage. After that, such digital signals pass by an analog-digital converter where the signal to be amplified returns to its original form, as electric variations analog to the sound pressure variations captured in the input. Finally, these electric variations are sent to an amplification circuit and from there they go to the receptor, where the amplified acoustic stimulations follow to the patient's internal acoustic meatus, through an acrylic or silicon mould.
The fitting provides the phonoaudiologist with full control of the hearing aid features, through these features recording and adjustments in the own hearing aid. In addition, it allows to file the patient's data in the computer so as to control, expand and adjust the hearing aid during its lifecycle (Picture 1).
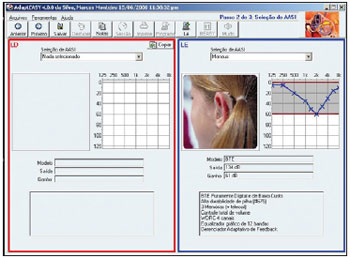
Picture 1. One of the AdaptEASY fitting screens.
Picture 2 shows the sequence used for the development of a digital hearing aid like Manaus, standing out the processes run on-line with SDT and processes run in the stand alone status.
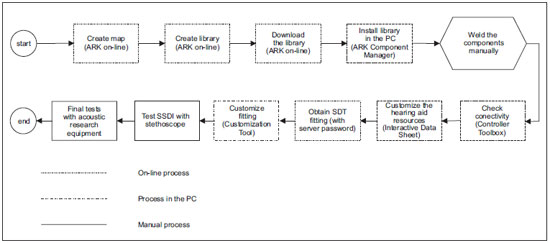
Picture 2. Manaus development process.
In order to evaluate the generic electronic circuit performance another Japanese technique was used, namely the Taguchi's Techniques applied in the quality engineering (17). These results have not been published so far.
RESULTSIn the following picture, we can observe the set mounted in exploded view, with focus on the generic electronic circuit components (Picture 3).
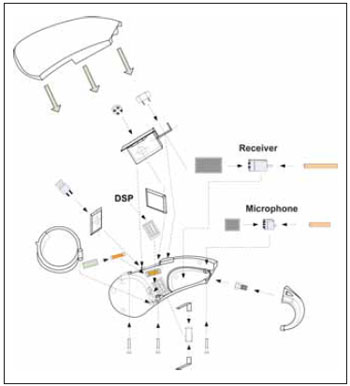
Picture 3. Manaus exploded view.
In the next picture, we can verify the internal aspect of a Manaus model BTE (Pictures 4 and 5).

Picture 4. Manaus model BTE in a final mounting step.
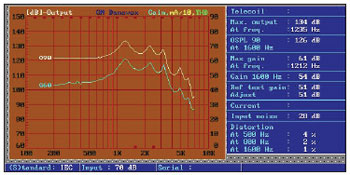
Picture 5. Screen of the equipment used for Manaus curves survey. Norm IEC 60.118-7.
Both the input and the output remained stable. The input noise parameter was limited to 28 dB and the greater distortion value obtained was 4% in 500 Hz. The drain current (not shown because there is no current measuring adaptor available) remained in 1 mA in the worst case.
The AdaptEASY fitting software enables the fitting in three simple steps, is easy to use, self-explained and also demand low hardware investments. It also allows for an unlimited number of patient's data entry. After connection, the AdaptEASY allows the patient's data entry (mainly personal and audiometry data); then it shows the hearing aid selection screen and in the last screen the AdaptEASY provides the phonoaudiologist with access to the hearing aid features, including the identification of the serial number of the hearing aids connected to him for traceability purposes.
Below (Picture 6) we can verify it in a block diagram simplified with the main components and their locations inside and outside the hearing aid.
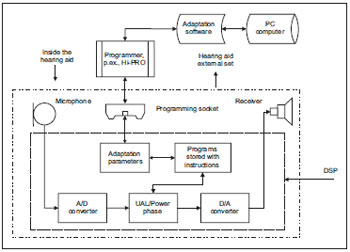
Picture 6. Manaus simplified blocks diagram
.
25 hearing aids, Manaus model, were mounted. The hearing aids presented the following results:
Output adjustable up to 132 dB; Stable input of up to 62 dB; Low battery consumption; Low harmonic distortion.The appliance offers the following features among others:
Blast fitting manager; (WDRC) Wide Dynamic Range Compression of 1, 2 or 4 canals; Graphic equalizer of 12 frequencies; Up to 4 comfort programs; Customizable filter for frequencies division adjustment (crossover) and knee point adjustment.The Manaus final value was of US$ 149.99.
DISCUSSIONThe worldwide market of hearing aids is supplied with Knowles, Sonion and Tibbetts trademarks transducers, and the last manufacturer has a few models intended for the hearing aid industry. This is the reason why we work with the other trademarks' transducers. However, we have opted for Knowles because it obtained the best results in a wide series of tests.
In order to develop a hearing aid that allowed for the mounting of a generic electronic circuit, we have kept contact with the national and international market suppliers that have credibility, quality and specially commit to take part in this project, which means they could supply samples, avail technical support and eventually international technical training in the basic and advanced levels and that could also supply this component to several hearing aids manufacturers. The hearing aid manufacturers search for the DSPs suppliers that supply the industry, whether they are SDT, DSP Factory, Etymotic Research, AMI Semiconductor, Resistance Technology or Texas Instruments. In addition, they should have a software or applications package as well as engineering tools for the development of customizable fitting product and program. It should also ensure the supply of components, even considering it is an academic work. SDT has been chosen for meeting the following requirements. Its DSPs are used by international companies, such as Audina, Electone, Bernafon, Magnatone, Unitron and Starkey, in addition to the national ones, such as Audifone and Amplivox. SDT also released in the second half of 2007 a new DSP that exceeded the features of GA3216 with slight difference in pricing. After the samples were requested, we mounted and tested some prototypes in laboratory. In February 2008, one of the researchers obtained advanced technical training on the GA3226 and the choice for this DSP was then made. All development of the GA3216 was then used for the GA3226.
The box is a low value added item, but the hearing aid manufacturers normally aim at offering designs advanced and different from the other manufacturers. We have chosen a standardized box from the In'Tech Industries supplier.
We sought to narrow the relationship with suppliers of the identified components, with which we could design and develop hearing aids with features and specifications not lower than those from international manufacturers. Among the features and specifications of the main component, we have chosen the DSP customizable for several designs of hearing aids so as to meet our generic electronic circuit, that is, one that allowed, from the same electronic configuration, to mount hearing aids in the BTE, ITC and CIC designs.
However the challenges to mount a hearing aid of a generic electronic circuit are numerous, among the following:
I. Functioning with feeding voltage of 1.35 VDC or less.
II. Having a current drain of approximately 1 mA or less.
III. The components must have reduced dimensions so as to allow the mounting of a CIC and ITC or BTE.
IV. The configuration must allow the build up of hearing aids that meet from slight losses up to the moderate-severe losses.
V. Functioning with a few features available to the patient (CIC) or even with a wide range of features (BTE).
VI. The fitting program must be able to recognize the different designs automatically, and among which, the different models, in addition to identifying the serial number of the hearing aid.
VII. The fitting program must be easily used by the phonoaudiologists.
VIII. Using standard programmers available in the market (HI-PRO and Microcard).
IX. Not demanding high hardware investments.
X. Using only two types of programming cables for all designs.
XI. This project hearing aids must not have performances lower that those traded in the Brazilian market, and have the same level of features.
XII. Having a low operational cost.
XIII. They must satisfy the patients in terms of quality of sound and available features, and also being easy to handle.
In the beginning low quality results were obtained, because the electronic configuration mounted had had low input and high levels of distortion, which resulted in low amplification quality realized even with the use of stethoscope. Even after with specifications both from SDT and Knowles and Sonion, the results took long to appear. It was necessary to promote several changes either in hardware (use of several combinations of transducers) through the ARK (changing delay times of microphone blasting manager, in the activation and answering times, compression rates change, in the three crossover filters frequencies, sample frequency etc.) or up to obtaining too positive results.
The test equipment used (CAS, of Danavox trademark) is dated of 1988 and limited to presenting reliable results, because among others, it doesn't respond to quick signals variations of a digital DSP with typical sampling frequency of 2.048 MHz.
From the project social importance viewpoint, we may simply observe in the chart below (Chart 1) the hearing aids imports in the last 19 years, according to data from the Ministry of Development, Industry and Commerce, obtained in 2008.
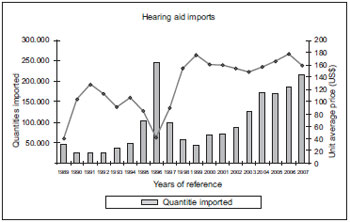
Chart 1. Imports declared in the period of 1989 through 2007.
Between 1989 and 1996 the average price was subject to huge changes, increasing in ascending line from 1997, remaining at the average of US$ 151.81. However, in 1996 the lowest valued declared of US$ 40.64 was reached, and allowed for a record in the hearing aids import. 245.559 units. Even with the maintenance of the high import prices, resulting in higher prices also for the hearing impaired people, the market reacted with a slow and gradual increase in the consumption, closing the year of 2005 with 169.575 units, that is, 69% of the total traded in the year of 1996. In 2006, the average price of a hearing aid was of US$ 177.87 and in 2007 the value dropped to US$ 159.35 - a diminishing of 10.4% - with imports reaching 214.310 units - increase of 16.7%. We can thus confirm that the hearing aids may be categorized as elastic demand products (18, 19) because their demand may be changed according to their prices. There is no factor identified to justify an ultimate price for a hearing aid of about R$ 4.400 or more for the hearing aids with the same features level as presented in this project.
The values declared in these imports don't comprise the hearing aid fitting services nor the warranty and the distribution costs, which are incurred to the importer and/or distributor. Even though, there are no basis to justify vehemently such a variation of values where services and products are united.
These manufacturers or importers' arguments there are high values involved in the imports payments for hearing aids and replacement parts are baseless, taking into account that the government exempts heavy taxes such as ICMS, IPI and part of the ISS from payment.
In the production management viewpoint, large manufacturers are benefited because they purchase several parts from their suppliers, which increase their bargain power with such suppliers and reduces inaccuracies in the product cycle performance measurements. Such manufacturers also have the advantage to compete in the international market, which results in having a great benefit regarding the several forms responded by the global market.
We can also discuss the "innovation power" these companies marketing attempts to comprise both for the professionals in the area and for the final customers. The feature of datalogging, the companies have recently sought to stand out in their products as being an "innovating" feature is in fact a feature that was present in the 3M models since the 80's when the present technology was still of analog programmables (23). Even the current and expensive hearing aids of Open technology were initially ranked as "over the counter" (product purchased without medical prescription) that cost $ 125 in Hong Kong, namely the Avance HE4 model by ReSound (24).
Killion and Gudmundsen stated in 2004 that "a hearing aid that meets slight to moderate losses could cost about $100 with the existing technology". As for them "there is a number of descomplicated auditory losses that don't demand sophisticated hearing aids" (25).
For the conducting of public policies, the Brazilian government uses the same classification as that of the Brazilian Academy of Audiology, presented through Decree 587 annex 5 of October 7, 2004, which sets up minimum criteria for each of the three technologies: A, B and C. Technology A comprises the simplest hearing aids, that don't need programming, have single channel compression and with a single memory. Technology B hearing aids must be programmable, with at least one channel WDRC and adjustment for the compression rate. Technology C must have multichannel WDRC, several memories, noise reduction algorithm and microphone blasting manager. The Government signalizes that technology A must compose 50% of its purchasing purpose, while technology B composes 35% and the remaining 15% are technology C hearing aids. This project's hearing aid meets technologies A, B and C requirements without any increase in price in order meet the specificities any technology, and it's only enough to customize the product for one technology or another. We can also export the product to less developed and developing countries specially in Latin America, Africa and Asia. Its manufacturing in Brazil will also contribute for the diminishing of import costs of sets traded in the national market.
The final value obtained from Manaus may be considerably reduced with the acquisition of its components in large scales linked to the manufacturing of hearing aids in large quantities. Because of the main benefit of producing low-cost digital hearing aids in a generic electronic circuit, the researchers have entered a patent application in INPI.
There is also inclusion of national labor with specialized training and capacity for inclusion of hearing impaired people across the productive process.
The hearing aids produced will promptly undergo clinical tests and be compared to the same category hearing aids.
CONCLUSION It was possible to produce programmable low-cost digital hearing aids in the BTE design with electroacoustic features equivalent to the hearing aids traded in the Brazilian market, which may make it a reference product for hearing habilitation and re-habilitation public policies.
BIBLIOGRAPHIC REFERENCES1. Raymann BCW, Béria JU, Gigante LP, Figueiredo AL, Jotz GP, Roithmann R, Costa SS, Garcez VR, Scherer CR. Perda auditiva incapacitante e fatores sócio-econômicos: um estudo de base populacional em Canoas, RS, Brasil. Universidade Luterana do Brasil, Canoas, capítulo de conclusão, em (2004).
2. Kochkin S. Hearing aids positively improve your quality of life. In: Carmen R. The consumer handbook on hearing loss and hearing aids: a bridge to healing. 2nd ed. Sedona, Arizona: Auricle Ink Publishers; 2004, p. 62-76.
3. Campos CAH, Russo ICP, Almeida K. Indicação, seleção e adaptação de próteses auditivas: princípios gerais. In : Fundamentos teóricos & aplicações clínicas. 2a ed. São Paulo: Lovise; 2003, p. 35-53.
4. Bento RF, Miniti A, Marone SAM. Tratado de otologia. 1a ed. São Paulo: EDUSP; 1998, p. 297-306.
5. Stack BA. Clinical audiology: An introduction. 1st ed. New York: Thomson Delmar Learning; 2004, p.465-480.
6. Lybarger SF. A historical overview. In: Sandlin RE. Handbook of hearing amplification. 2nd ed, vol.1.San Diego,California: Singular Publishing Group;1988, p. 1-27.
7. Redondo MC, Filho OCL. Testes básicos de avaliação auditiva In : Tratado de fonoaudiologia. 1a ed. São Paulo: Roca; 1997, p. 83-107.
8. World Health Organization - Prevention of blindness and deafness. Guidelines for hearing aids and services for developing countries. 2nd ed.; sept. 2004.
9. Guinta LR, Praizler NC. Manual de QFD. 1a ed. Rio de Janeiro: LTC; 1998, P. 41-59.
10. GA3226 Getting started with GA3226 digital. Burlington, Canada, doc. 44812, july 2007.
11. GA3226 Preliminary data sheet, Sound Design Technologies. Burlington, Canada, doc. 42661-0, july 2007.
12. Digital hybrids reference designs. Burlington, Canada, doc. 31840, july 2007.
13. Getting started with Foundation digital. Burlington, Canada, doc. 25786, feb. 2004.
14. Components selection in hearing aids. Burlington, Canada, doc. 13345-8, nov. 2007.
15. Knowles microphone data sheet, Itasca, IL, doc. M10101453, april 2007.
16. Knowles receiver data sheet, Itasca, IL, doc. C10103246, oct. 2005.
17. Ross, Phillip. Aplicações das técnicas de Taguchi na engenharia da qualidade. Edição não informada. São Paulo: Mc Grow Hill; 1991, p. 74-158.
18. Porter, M. Estratégia Competitiva - Técnicas para Análise da Indústria e da Concorrência. 2a Ed. São Paulo: Campus; 1988, p. 22-47.
19. Ghemawat, P. Strategy and the business landscape. 2nd ed. Upper Saddle River, New Jersey: Pearson Prentice Hall; 2006; p. 17-28.
20. Machado MC, Toledo NN. Gestão do processo de desenvolvimento de produtos - uma aboragem baseada na criação de valor. 1a Ed. São Paulo: Atlas; 2008; p. 10-23.
21. Donaire D, Penteado SP. Uma análise da lucrativa indústria de aparelhos auditivos e a apresentação de uma solução tecnológica de baixo custo. Revista Brasileira se Ciência da Saúde. 2007, 12-21.
22. Christensen CM. O dilema da inovação. Edição não informada. São Paulo: Makron; 2001, p.32-60.
23. Stypulkowski PH. 3M programmable hearing instruments. In: Sandlin RE. Handbook of hearing amplification. 2nd ed, vol.1. San Diego,California: Singular Publishing Group;1988, p. 97-116.
24. McPherson B, Wong ETL. Effectiveness of and affordable hearing aid with elderly people. Dis. and Reab. 2005, 27(11):601-609.
25. Zimmermann A. Sound and fury: the noisy debate over hearing aids: why so expansive? Pioneer in field think they should sell over the counter; FDA backs specialists' role; device for hunters works fine. In: Wall Street Journal (eastern edition). New York, N.Y.: mar. 24, 2004. p.A1.
1. Electronic Engineer by the Pontifical Catholic University of São Paulo. Student of the Doctoral Course in the FMUSP's Otorhinolaryngology Program.
2. Titular Professor of the Otorhinolaryngology Discipline of the Medicine College of University of São Paulo.
Institutions: Medicine College of the University of São Paulo and Otorhinolaryngology Foundation. São Paulo / SP - Brazil
Mail address:
Silvio Pires Penteado
Avenida Dr. Enéas Carvalho de Aguiar, 256 - 6º Andar - Sala 6167
São Paulo / SP - Brazil - Zipcode: 05403-000
Telephone: (+55 11) 3069-7834
E-mail: penteado@usp.br
Article received on July 27, 2008.
Approved on September 23, 2008.