INTRODUCTIONThe labiopalatine clefts are the most common craniofacial congenital malformations, and they occur in 1 at each 757,5 births and in 1 at each 923,53 newborn alive (1) Especially the lip clefts, with palate commitment or not, affect more the masculine sex and have a variable incidence among the different ethnic groups, and the Asian have major risk. The clefts palates without lip commitment have homogeneous incidence among the ethnics and occur more in the feminine sex (2).
There are some classifications used to categorize and describe the type of labiopalatine cleft anatomically. In the HCPA's Craniomaxillofacial Plastic Surgery Service, we used the Kriens' classification (3), known as LAHSHAL. It is a system composed by seven digits that allows for the description of the cleft shape, including microforms, by using capital letters to describe the complete forms and small letters for the incomplete ones. Therefore, we have "L;I" corresponding to the lip, "A;a" for alveolus, "H;h" representing the hard palate (from the English, hard) and "S;s" for the soft palate (from the English, soft). The reading is made from patient's right side to the left, for example, LAHS, corresponding to the patient's right side complete labiopalatine cleft. The microforms are described with asterisk (*), replacing the corresponding letter (Picture 1).
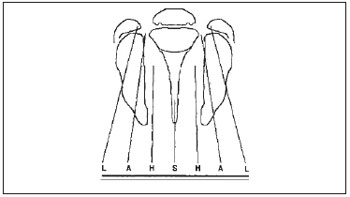
Picture 1. Documentation system "LAHSHAL": L = Lip; A = Alveolus; H = Hard palate; S = Soft palate. (Collares; Westphalen; Costa & Golgin. Retirado da Rev. AMRIGS, Porto Alegre, 39 (3); 183-188, jul, ago/set. 1995).
Despite the Kriens' classification allows for a fast understanding of the cleft type and facilitates the communication among the multidisciplinary team professionals, it's not commonly used in the researches about the theme. Therefore, the Spina's (4) classification is predominantly found, which uses as a reference point the incisive foramen to limit the area affected and name the type of cleft. The clefts that involve the lip and the dental alveolus, as well as the hard palate frontal portion, are classified as pre-foramen clefts, and may be unilateral or bilateral. The incisive post-foramen clefts involve the remaining parts of hard palate and soft palate. The clefts that involve both incisive pre-foramen and post-foramen regions are called trans-foramen.
The aesthetic and functional changes found are several in the labiopalatine clefts. The multidisciplinary treatment is an indispensable condition for the success of these patients rehabilitation. The treatment center must count on several professionals: plastic surgeon, otorhinolaryngologist, phonoaudiologist, orthodontist, psychologist, geneticist, paediatrician etc. All these professionals, whether or not they represent a reference center in the treatment of clefts palates, must bear in mind, upon receiving a cleft palate patient for the first time, a wide spectrum of functional changes present. We are not only faced with a visible change in the palate, which makes the newly-born child's feeding difficult, nor even with an evident aesthetic change in the lip cleft: there is a whole complex network of functional changes in the deglutition, speech, hearing standards, craniofacial development and growth, nasal breathing. The early diagnosis and management are fundamental to prevent undesirable sequels in all these aspects.
The understanding of the physiology in the affected craniofacial structures in the case of labiopalatine clefts is fundamental to comprehend and guide the best therapeutic modalities. The (EVF) velopharyngeal sphincter region is, perhaps, the most critical point for the understanding of the functional changes pathophysiology that occur in this malformation.
The EVF is a muscular belt located between the oro-nasal and nasopharynx, comprising the muscle of the soft palate and the lateral and frontal walls of the pharynx. Out of these muscles, those of the soft palate and specially of the palatine veil tensor, have a primary function in this region physiologic maintenance. There is a close relationship of this muscular grouping with the Eustachian tube, with insertions in the very tube cartilage and at the basis of the adjacent cranium.
In this review article, a bibliographic research was performed online, as well as in books and periodicals regarding the aspects relating to the velopharyngeal sphincter. LILACS, MEDLINE and SciELO databases were researched, by applying to the research the interest terms, all relating to the velopharyngeal sphincter physiopathology, for articles published between 1965 and 2007. The literature examined approaches physiological aspects that specifically involve the velopharyngeal sphincter. Such functional changes are relevant due to their consequences in the patient's development and socialization.
Deglutition pathophysiology in the labiopalatine cleftsThe deglutition, an integral function of the stomatognatic system, has as its main objective the propulsion of the bolus, liquid or saliva, from the oral cavity to the stomach. It also has the function of protection when it removes particles originally lost in the nasopharynx or when returns the digestive material that refluxes from the stomach to the esophagus or pharynx. From the thirtieth second week of intrauterine life, it is possible to observe suction and deglutition, functions that provide a balance in the amniotic liquid volume, allowing for the neurological system integrity. The fetus needs to perform this function early to stimulate the adequate growth of the medium third of the face, and ensure the success in the breathing process at birth (7, 8, 9).
The deglutition is a continuous action, but may be physiologically divided in four different phases: pre-oral, oral, pharyngeal and esophageal. The pre-oral phase qualifies the food to be swallowed or eliminated from the oral cavity, and has an execution time ranging according to the consistency, volume and will or need for the individual to manipulate the intra-oral bolus. This phase counts on the participation of chewing muscles, innervated by the mandibular branch of the trigeminus nerve (V-3), the lips sphincter and facial musculature, innervated by the cranial pair VII, and the tongue with performance of the intrinsic and extrinsic musculature, innervated respectively by pair XII and cervical strap (C1-C2) and the hard palate, commanded by pair X. The lips, tongue, dental arch, hard palate and the cheeks perform a fundamental synchronic motion in this step, and they need tissue integrity for a satisfactory performance. The labial sealing becomes necessary so as to prevent the oral escape of the food, as well as the soft palate remains in a lower position in order to avoid the bolus early escape towards the pharynx. In the labiopalatine clefts there are changes in this function physiology, mainly in cases which no surgical intervention has occurred, in the dental structures severe commitments, in the presence of palate fistulas and excess of fibrous tissue. The mentioned food loss may occur because of reduction in the labial sealing or exacerbated protrusion of the tongue, for inefficient chewing compensation (5, 13, 22).
The deglutition oral phase starts with the bolus voluntary propulsion towards the oropharynx and is considered by some authors to be a transition between the oral preparatory and pharyngeal phases. The tongue conditions the bolus, by pushing it backwards and in this dynamics the time interval between its contact with the hard palate is of only one second. This phase composes a very important physiologic event for the process continuity, which is the unlocking of the deglutition reflex, necessary for the transition to the pharyngeal phase. Touches or pressure stimulation occurred mainly in the frontal amygdaline pillars may unlock the deglutition, as well as sensorial responses also in the soft palate, uvula, dorsum of tongue, epiglottis pharyngeal surface, pyriform sinuses, pharynx back wall and pharyngoesophageal articulation. Therefore, there are several sensors that may unlock the deglutition Pharyngeal Phase in the oropharynx. It involves a complex and integrated sequence of physiologic events that provide an adequate passage of the food to the esophagus and also prevent the bolus to divert and go to the nasopharynx or penetrate the bronchial tree.
The pharyngeal phase involves the elevation of the soft palate and constriction of the pharynx back wall, under innervation of cranial pairs V, IX and X, elevation of the tongue, under command of pairs VII and XII, followed of the larynx elevation and frontal misbehavior, as a response to the stimulus of pairs IX and X (22, 5).
When the (EVF) Velopharyngeal sphincter structures, for different reasons, do not work correctly, there is a velopharyngeal unfitness that brings as a consequence a gap between such structures, and allows the communication between the cavities of the oropharynx and nasopharynx, by means of the region closure command. This change symptomatology is diversified in the phonation, feeding and hearing functions. In the deglutition there may occur chokes and nasal refluxes. The velopharyngeal occlusion strength maximum point, during deglutition, tends to be lower than that observed in the speech, probably by the action of the deglutition constricting muscles (25, 26).
In addition to the anatomic characteristics, the EVF occlusion variability also depends on the action performed, therefore, when the activity is pneumatic (blowing, speech, whistle), the pharynx side walls do not move in its whole extension. In the other hand, during non-pneumatic activities (deglutition, suction and vomit reflex), these walls motion is full, in all of their extension (27). In the deglutition pharyngeal phase, the nasal escape is prevented by the soft palate adjustment against the pharynx back wall, thus avoiding the pressure dissipation.
The larynx cranial displacement, along with the cricopharyngeal muscle, generates a low pressure zone in the hypopharynx that contributes for the food propulsion and also provides the opening of the (EES) upper esophageal sphincter, which allows for the passage of the bolus to the esophagus. Therefore, there occurs the transition from the pharyngeal to the esophageal phase. After the passage of the bolus through the EES, the larynx returns to its normal position and the sphincter muscular tension increases, and prevents regurgitation and aerophagy. The esophageal transport involves the bolus peristole in the direction of the caudal-cranium, finalizing with the lower esophageal sphincter relaxation and the passage from the esophagus to the stomach (9,10). The labiopalatine clefts may be isolated congenital anomalies or part of a syndrome or association, and thus imply a careful investigation in search of other changes. The esophageal atresia may be an anomaly associated to the labiopalatine cleft in about 15 to 20% of the cases and such anatomic condition may lead to changes in the deglutition esophageal phase (28).
Moyers (11) subdivided the normal deglutition according to the development period, and called them child or visceral deglutition and mature or somatic deglutition. The first one is characteristic of the newborn and is manifested by the interposition of the tongue between the alveolar borders and the lips, and is considered normal until the eruption of the first deciduous molars. If it persists, it gets around from the normality standards and begins to be considered atypical. The second one is characterized by the positioning and pressure of the top of the tongue towards the alveolar process, behind the upper incisives.
Neves et al. (12) carried out a bibliographic review on dental anomalies in labiopalatine cleft patients and came to a conclusion that these are evidenced more in these individuals then in non-cleft individuals, as well as occurs with major incidence in the permanent dentition, in the cleft region. The most frequent dental anomalies observed in porters of labiopalatine cleft are anadontia, supernumerary teeth, ectopic eruption, formation delay and dental eruption.
The tongue performs a fundamental role in the deglutition process effectiveness and its suitable position in this physiologic dynamics is in the dependence upon their dimensions relating to the width and length of the maxillary bone, the hard palate anatomy, standing out, inclination or torque of the upper incisives in the vestibulolingual direction (13). A study performed by Faraj (14) on dimensional changes of the dental arches in cleft-affected children, in the complete deciduous dentition phase, shows that their maxillary dental arch presented major changes in the cross direction when compared to the group of non-deformity children, with more significant changes in the bilateral labiopalatine cleft. Therefore, the tongue positioning in rest or in oral functions is committed and many times it becomes compensatory.
In the individuals that have structure and/or function disorders, such as, in the labiopalatine clefts, the atypia found in the deglutition standard is a consequence of the search for adaptation, called adapted deglutition by some authors. In case of osseous changes in the face, that cause asymmetries, it is also possible there is a tongue inadequate positioning caused by the change of the internal space of the bucal cavity. (7, 15, 16, 17).
Bilton e Lederman (18) and Rocha (19) quantify the musculature taking part in the activities comprised by the deglutition in thirty muscles and six pairs of cranioencephalic nerves, which differs from Macedo Filho (20), who considers the participation of forty-eight muscles. The deglutition action occurs in an average of 600 times, and reaches two thousand times a day in a healthy adult man, and it is a complex and coordinated biological function, integrated to the central nervous system that has a synergic and opposite mechanism of muscular actions led by reflex-arches (21, 22,8). The involved structures skeleton muscle changes lead to changes in the function performance, and search for compensatory adaptation standards, as in the case of the labiopalatine clefts.
The feeding difficulty of the child porting labiopalatine cleft may interfere with the global development process, as well as in the schedule for surgical steps, which generally depend on the nutrition daily allowance and their proper physical development. The most significant obstacles occur in the cases of pos-foramen or incisive transforamen, resulting from inadequate intraoral pressure (23).
Pathophysiology of the Eustachian tube and the middle ear in the cleft palateThe Eustachian tube is the osteocartilaginous channel, internally coated by breathing mucosa, which communicates the middle ear with the nasopharynx light. The osseous portion represents the channel upper third, which is permanently open and has its end at the middle ear. The two remaining thirds are of fibrocartilaginous constitution, have a virtual lumen and their caudal end is the opening in the nasopharynx, the tube torus. At each deglutition or yawn there occurs a brief opening of the lumen of the tube cartilaginous portion that allows for the air passage to the middle ear.
During most of the time the tube remains closed, preventing the reflux of secretions and bacteria from the nasopharynx to the middle ear. There is also a mucociliary beating mechanism towards the nasopharynx that prevents such reflux. The tube closing also protects the middle ear from floatings in the nasopharynx pressure during the cough, breathing, deglutition and Valsalva (29).
The middle ear is an airy space limited by osseous and therefore non-deformable walls; the only exception is the lateral wall, the tympanic membrane, that is flexible. It is fundamental, in the pathophysiology study, to have the understanding of the middle ear airy space as a single cavity in continuity with the mastoid cells with which it communicates through the aditus. Therefore, the set of mastoid cells, middle ear and osseous portion of the Eustachian tube form a single gas bag that may be called hearing vent. The periodic opening Eustachian tube light equals the atmospheric and middle ear pressures, preventing the maintenance of a cycle that leads to negative pressures typical of hypoventilated hearing vent in the tube disorder.
The cleft palate association with the middle ear diseases is very known and studied. The almost universality of the otitis media with effusion in breast-fed infants with cleft palate was described by several authors since Paradise et al (30), in 1969, identified 97% of the prevailing of such change in children from 2 months to 2 years of age with the cleft.
The main reason for the occurrence of the otitis media with effusion in children with cleft palate seems to be the tube chronic disorder, specially for a failure in the tube opening mechanism. There is suggested to be a correlation between tube disorder and otitis media with effusion in the childhood and the colesteatomatous and non-colesteatomatous chronic otitis media, and hearing loss (31). Such connection between the hearing vent reversible changes and the tissue damage chronic forms was studied by Paparella (32) and was called continuum theory. This theory is interconnected to several forms of otitis media as a way to explain its pathogenesis, and shows that the chronic otitis media, that with effusion (serous secretion), the colesteatomatous and non-colesteatomatous chronic otitis media are, actually, successive phases of the same nosologic entity and not distinct diseases. Progressive tissue events and changes occur in the dependence on the maintenance of an unlocking factor.
The persistent Eustachian tube dysfunction is the initial event in this evolutive cascade of otites media that, with a pathologic maintenance process, tends to evolve from the minor to the major severe and irreversible forms of tissue changes. The understanding of the Eustachian tube physiology may help to understand the reason for this tube dysfunction prolonged in the patient with cleft palate.
Two main mechanisms were studied as the cause for the Eustachian tube opening: the exclusive contraction of the palatine veil tension muscle, that has its insertion in the tube membrane lateral portion (33, 34); and the joint contraction of the palatine veil tensor and elevator, spinning and opening the tube lumen (35). For both mechanisms, a suitable palatine veil tension muscle contraction is needed to effectively expand the tube lumen.
In the cleft palate the palatine veil tensor and elevator muscles have an important change in their traffic and insertion in the palate, since there is no occurrence of the union between the muscular fibers of both sides in the medium line of the soft palate; quite the contrary, the insertion occurs in the cleft ipsilateral hard palate. Therefore, the muscular contraction tends to be defective and the traction normally expected of the Eustachian tube cartilage does not occur.
The palatoplasty seems to have a positive effect in the otologic disease, by preventing the development of the (OME) otitis media with effusion, or even solving the OME already established. Several studies show the benefits of the palatoplasty in the reduction of the otitis media frequency in the labiopalatine clefts patients (36, 37, 38). The role of the palatoplasty in the reduction of the unfavorable otologic conclusions was studied by Kruse (39), who showed a trend for decrease of these events in the school age in those patients who underwent early palatoplasty in their lives (before the 24 months). The palatoplasty technique has also been evaluated by several authors. Hassan & Askar (40) recently studied 70 patients as for the suitable or non-suitable reconstruction of the velopalatine musculature and showed better conclusions compared to the tube and velopharyngeal function in that group submitted to reconstruction.
However, histopathological studies have been showing changes in the velopharyngeal musculature, not only in the soft palate region and velopharyngeal sphincter, but also in their Eustachian tube insertions, as well as structural changes of the muscle and in the very tube cartilage. An abnormal traffic or insertion of the palatine veil elevator or a palatine veil tensor hypoplasy have also been reported with possible mechanisms responsible for the chronic tube dysfunction in the cleft palate (41, 42, 43, 44).
Matsune et al (45) studied 30 temporal bones (10 of cleft palate children and 20 normal controls) and found changes in the palatine veil tensor in the tube cartilage in 4 of 10 cleft palate temporal bones; in theses cases the muscle practically has no insertion or is inserted in a very reduced area of the tube. Even in the other 6 cleft bones, the muscle insertion seemed to be weaker or minor than in the control group. Sehhati-Chafai-Leuwer et al (46) evaluated through magnetic nuclear resonance the velopharyngeal musculature of 15 adult patients with corrected cleft palate, and studied the simultaneous presence of chronic otitis media and came to the conclusion that the palatine veil and pterygoid hamulus tensor muscle are crucial for the otologic conclusion.
Therefore, the abnormalities of the palatine musculature in the patient with vent seem to influence the tube function both by their abnormalities inherent to the muscle masses separation in the palate middle line as due to the changes in the insertion of such masses in the Eustachian tube.
The presence of high prevalences of otites media in the population of patients with cleft palates makes it a very vulnerable group in terms of hearing health. It is known that otitis media with effusion in the childhood is responsible for a hearing loss of conductive type, of a reversible grade from light to moderate. As this change is almost universal in breast-fed infants with cleft palate, we also observe a high prevalence of hearing loss in this population. This group must be treated early and we must always recall that a perfect hearing system is fundamental for the acquisition of the spoken language: it is right in the period in which the child acquires this language that we observe otitis media with effusion and its consequent hearing loss.
The most early forms of otitis media, still considered as a reversible process of tissue damage, represented basically by tympanic retractions in the middle ear, include hearing loss equally reversible. The most severe chronic forms of otitis have an inherent trend for progressive and irreversible hearing loss, when not treated properly. Therefore, the diagnosis and treatment of otitis early forms, specially with insertion of ventilation tubes, seem to be the main tool for the prevention of hearing loss and its functional, social and psychological consequences.
According to Carvalhal (47), the prevalence of the otitis media reversible forms is high until the six years of age in patients with palatine vent. From this moment on, supposedly due to the craniofacial and Eustachian tube development, there is a dramatic decrease of the reversible forms prevalence and simultaneously an increase of the prevalence of the otitis media chronic forms, with irreversible tissue changes. More than once the early diagnosis and intervention by the otorhinolaryngologist is justified to prevent the middle ear disease chronification in the patient with cleft palate.
Pathophysiology of the speech in labiopalatine cleftThe speech is produced by three mechanisms that act by means of polyphasic and sequential motor actions associated with breathing, larynx (source of sound energy) and the supraglottic cavities (cavities of nose and oral cavity) that have resonators' function. (48). The sound waves are originated by the vocal chords vibration, but other structures are necessary to transform the sound into recognizable speech. The pharynx, nasal cavity and oral cavity (resonance chambers) are responsible for intensifying and amplifying the sound generated by the vocal chords. In the production of vowels there is a constriction and relaxing of the muscles in the pharynx walls and the muscles of the face, lips, the alveolar process and teeth contribute for the words to be pronounced (49, 50). Also relevant for the production of normal speech is the velopharyngeal function that needs the velopharyngeal mechanism structures motions synchrony (soft palate, side walls and the pharynx back wall) that is responsible for the distribution and forwarding of the exhaled air flow and the acoustic vibrations to the oral cavity, in the oral sounds and to the nasal cavity, in the nasal sounds. (51).
Altmann e Lederman (52) verified that in the videofluoroscopy exam it is possible to view the mechanism and the anatomic characteristics of the velopharyngeal sphincter, which shows differences of size between adults and children (the veil is shorter). In the same study it was possible to verify that in the speech there is a major displacement of the pharynx side walls that shows 50% of its extension in rest.
The velopharyngeal mechanism is a muscular valve that expands from the hard palate to the pharynx back wall and is located in the portion of the vocal tract called velopharynx. The patient with labiopalatine cleft may have changes of the velopharyngeal mechanism damaging the speech intelligibility, that is, when there is not the suitable closing of the velopharyngeal sphincter the air flow gets around by the nasal cavities. The term velopharyngeal dysfunction is used to express its inadequacies resulting from the lack of the soft palate tissue to complete the correct velopharyngeal closing (velopharyngeal insufficiency) or the neuromuscular incompetence with the velopharyngeal structures motion (velopharyngeal incompetence) (53, 54).
The structural abnormalities resulting from the cleft palate reflect some changes in the speech and the most common ones are associated to the velopharyngeal dysfunction, such as hypernasality, the emission of audible air and the articulatory and compensatory disturbances. The occlusal and dental deformities may also damage the phonemes articulation and because of this reduce the speech understanding (56).
The hypernasality is one of the speech symptoms resulting from the velopharyngeal dysfunction, where the oral phonemes nasal resonance occurs for the lack of sealing between the oral and nasal cavity (57). According to Dworkin et al (49) the hypernasality is the main perceptually auditive characteristic evident in the speech of individuals with clinically significant velopharyngeal dysfunctions. These researchers also explain there is a strong correlation between the hypernasal speech resonance severity and any emissions of consonants that require a high intraoral pressure for its normal production. Hanayama and cols. (58) describe that the most frequent causes of nasality are the palatoplasty sequels, cleft palate correction surgery, followed of the submucous clefts. Scherer (59) mentions that even if the individual undergoes surgical repair in the first two years of age, in some cases a residual velopharyngeal dysfunction may occur in the post-operative period associated to the hypernasality due to different unexplainable complications.
Generally, the major the velopharyngeal sphincter gap, the more perceptible the hypernasality may be. However, even the patients with reduced velopharyngeal closing area, the hypernasality may be very strong (58, 60).
In children with velopharyngeal dysfunction, as occurs in cases of labiopalatine clefts, we can normally observe a major effort in the production of consonants that demand high intraoral pressures, such as the fricative ones (example: /f/-
force],/v/-
vision), africate (example: /tò/-
tie, /dò/-
day ) and plosive (example: /p/-
pay, /b/-
boom). These articulatory changes types in the class of phonemes tend to be variable and may include distortion, substitution and omission errors. As the vowels furnish the acoustic power source in the speech, they are susceptible to be articulated with an excessive nasal air flow and characterized of hypernasal resonance, specially in the following consonants K, T, S, F. This clinical population may also have facial mimics and nostril constriction during the speech. The scenarios described occur intentionally, that is, are intended to recruit the facial muscles in an attempt to reduce the nostrils opening dimensions and restrict the nasal air flow dynamics excess.
The articulatory changes in the labiopalatine cleft patients may be synthesized in: nasal snore, nasal lisp, mandibular compensation, light articulatory contacts, mid-dorsum palatal compensation, pharyngeal fricative and glottis cut (52). The individuals submitted to the cleft palate repairing surgery may still have reduced speech intelligibility (51).
The phonatory anomalies resulting from the velopharyngeal dysfunction and hypernasal resonance may cause notorious vocal difficulties in the cleft palate patients in the attempt to conduct the voice with a major breathing and laryngeal effort to compensate the vocal intensity deficiency. Such excessive effort is seldom beneficial. In the other hand, the prolonged use of such behavior may lead to the development of disphony for muscular tension and anomalies of vocal chords, such as, nodes and generalized edema, that result in raucousness (48).
The labial and/or palatal clefts are characterized by anatomic deformities of variable extensions of lips, alveolar process, hard and soft palates associated to the velopharyngeal mechanism dysfunctions. Such structural abnormalities comprise the production and intelligibility of the speech. It's important to recall that the health professionals must be able to detect the anatomic changes and understand their consequences in the several affected functions, such as the speech.
FINAL CONSIDERATIONSAt the end of the bibliographic review about the velopharyngeal physiology in individuals carrying labiopalatine clefts in the functions of speech, deglutition and otologic functions, it was possible to observe that despite the literature pursuant to the theme is wide, some pathophysiological aspects must be more deeply studied.
The cleft palate association with the middle ear diseases is very known and studied. There is a huge prevalence of otitis media with effusion in the breast-fed infants with consequent reversible hearing loss. After the six years of age there occurs an increase of the otitis media chronic forms with major possibility of irreversible sequels with chronic infections and hearing loss. The early intervention role is not yet defined in the literature, with palatoplasty and insertion of ventilation tubes for the prevention of such sequels, but there seems to be a positive effect of such early conducts in the life of the patients.
We observed in the examined literature that the deglutition physiology characterization in labiopalatine cleft patients is restrict, mainly in terms of oral and pharyngeal phases. There is a wide database regarding the initial difficulties of these individuals, with emphasis in the breast-feeding process, suction function and nasal reflux of food. In the other hand, the clinical practice reveals that in patients older than 6 years of age, already operated, the complaints regarding deglutition are minimal, even in those with a significant gap in the EVF closing. The functional description of the accomplished compensation is confusing, as well as the reference to atypical signals in the food transit course in the phases described is limited.
In the other hand, the pathophysiology of the speech of labiopalatine cleft affected is well described and referenced in the literature. The compensatory mechanisms have a specific characterization, including evaluation standardized methods so that they are better observed. Therefore, the studies also contribute for a more specific therapeutic approach for each compensatory condition detected.
At last, the early diagnosis and evaluation with specialized professionals for the suitable treatment are fundamental in the management of these patients, by always searching for a multidisciplinary service where all aspects of the disease are taken into account.
BIBLIOGRAPHICAL REFERENCES1. Collares MVM, Westphalen ACA, Dalla Costa TC, Goldim JR. Fissuras lábio-palatinas: incidência e prevalência da patologia no Hospital de Clínicas de Porto Alegre. Um estudo de 10 anos. Rev Amrigs. 1995, 39(3):183-8.
2. Modolin M, Kamakura L, Cerqueira EM. Classificação, etiologia, patogenia e incidência das fissuras labiopalatinas. Em: Carreirão S. Tratamento das fissuras labiopalatinas, 1996.
3. Kriens O. Documentation of cleft lip, alveolus, and palate. In: Bardach J, Morris H eds. Multidisciplinary management of cleft lip and palate. Philadelphia: Saunders; 1990, pp. 127-33.
4. Spina, V, Psillakis JM, Lapa FS, Ferreira MC. Classificação das fissuras labiopalatinas: sugestão de modificação. Rev Hosp Clin Fac Med S Paulo. 1972, 27:5-6.
5. Marchezan, IQ. Deglutição - Normalidade. Em: Furkin, AM e Santini, CS (eds). Disfagias Orofaríngeas. Carapicuíba, Pró-Fono; 1999, pp. 3-18.
6. Reid J, Reilly S, Kilpatrick N. Sucking performance of babies with cleft conditions. Cleft Palate Craniofac J. 2006, 44(3):312-320.
7. Marchezan IQ, Junqueira P. Atipia ou adaptação: como considerar os problemas de deglutição. Em: Junqueira P, Dauden ATB (eds). Aspectos atuais em terapia fonoaudiológica. 2ª ed. São Paulo: Pancast; 1997, pp. 11-23.
8. Ribeiro, LMM. Deglutição: processo normal e patológico. Monografia (Especialização em Motricidade Oral). Centro de Especialização em Fonoaudiologia Clínica. Londrina; 2000, pp. 38.
9. Jotz GP, Dornelles S. Fisiologia da Deglutição. In: Costa, SS (eds). Otorrinolaringologia: princípios e práticas. 2ª ed. Artmed. Porto Alegre: Artes Médicas; 2006, pp. 753-756.
10. Yamada EK, Siqueira KO, Xerez D, Koch HA, Costa MMBA. Influência das fases oral e faríngea na dinâmica da deglutição. Arq Gastroenterol. 2004, 41(1):18-23.
11. Moyers, R. Ortodontia. 4ª.ed. Rio de Janeiro: Guanabara-Koogan; 1991.
12. Neves ACC, Patrocínio MC, Leme KP, Ui RT. Anomalias dentárias em pacientes portadores de fissuras labiopalatina: revisão de literatura. Revista Biociências. 2002, 8(2).
13. Molina OF. Fisiologia Craniomandibular: oclusão e ATM. São Paulo: Pancast; 1989. pp. 44-49.
14. Faraj JORA. Alterações dimensionais dos arcos dentários em fissura lábio-palatina. Bauru, 2006, (Dissertação de mestrado - Universidade de São Paulo).
15. Mazzotini R. Variações nas dimensões do arco dentário superior em fissurados unilaterais, em função da época de tratamento cirúrgico. Bauru, 1985, (Tese de Doutorado - Universidade de São Paulo).
16. Araújo SOA. A língua e a deglutição. Fortaleza, 2001. Monografia de conclusão do curso de Especialização em Motricidade Oral. Centro de Estudos Fonoudiológicos.
17. Cleall FJ. Deglutition: A study of form and function. Am J Orthodontics. 1965, 51(8).
18. Bilton TL, Lederman HM. Anatomia, fisiologia e normatização da videofluoroscopia da deglutição. Em: Marchezan IQ, Zorzi JL, Gomes ICD (eds). Tópicos em Fonoaudiologia. São Paulo: Lovise; 1998, vol. 4, cap. 15, pp. 261-265.
19. Rocha EMSS. Disfagia: avaliação e terapia. Em: Marquezan IQ. Fundamentos em fonoaudiologia: aspectos clínicos da motricidade oral. Rio de Janeiro: Guanabara Koogan; 1998, pp. 51-57.
20. Macedo Filho ED. Conceitos e fisiologia aplicada da deglutição. Em: Macedo Filho ED, Pissani JC, Carneiro J, Gomes G (eds). Disfagia: Abordagem multidiscipinar. 2ª ed. São Paulo: Frôntis; 1999, pp.3-8.
21. Ferraz MCA. Alterações oromiofaciais. Em: Araújo R, Pracownik A, Soares L (eds). Fonoaudiologia Atual. Rio de Janeiro: Revinter; 1997, cap. 9, pp.63-65.
22. Douglas CR. Patofisiologia oral: fisiologia normal e patológica aplicada à odontologia e fonoaudiologia. São Paulo: Pancast; 1998, p. 273-277.
23. Araruna RC, Vendrúscolo DMS. Alimentação da criança com fissura de lábio e/ou palato: um estudo bibliográfico. Rev Latino-Am Enfermagem. 2000, 8(2):99-105.
24. Keller JT, Saunders MC Van Loveren H, Shipley MT. Neuroanatomical considerations of palatal muscles; tensor and elevator veli palatine. Cleft Palate J. 1984, 21(2):70-5.
25. Tabith Jr A. Contribuição ao estudo da insuficiência velofaríngea. São Paulo, 1989 (Dissertação de Mestrado - Pontifícia Universidade Católica de São Paulo).
26. Mc Connel FN. Analysis of pressure generation and bolus transit during pharyngeal swallowing.Laryngoscope. 1988, 98(1):71-8.
27. Altmann EBC. Anatomia e fisiologia do esfíncter velofaríngico. Em: Altmann EBC (eds). Fissuras labiopalatinas. 2 ed. São Paulo: Pró-fono; 1997, pp.156-133.
28. Ribeiro EM, Moreira ASCG. Atualização sobre tratamento multidisciplinar das fissuras labiais e palatinas. RBPS. 2005, 18(1):31-41.
29. Monsell EM, Harley RE. Eustachian tube dysfunction. Otolaryngol Clin North Am. 1996, 29(3):437-444.
30. Paradise JL, Bluestone CD, et al. The universality of otitis media with effusion in 50 infants with cleft palate. Pediatrics. 1969, 44:35-42.
31. Tos M. Uppon the relationship between secretory otitis in childhood and chronic otitis and its sequelae in adults. J Laryngol Otol. 1981, 95:1011-22.
32. Paparella MM, Hiraide F, Juhn SK, Kaneco J. Celular events involved in middle ear fluid production. Ann Otol Rhinol Laryngol. 1970, 79(4):766-79.
33. Rood SR, Doyle WJ. The nasopharyngeal orifice of the auditory tube: implications for tubal dynamics anatomy. Cleft Palate J. 1982, 19:119.
34. Casselbrant ML, Cantekin EL, Dirkmaat DC, Doyle WJ, Bluestone CD. Experimental paralysis of the tensor veli palatini muscles. Acta Otolaryngol (Stockh). 1988, 106:178.
35. Proctor B. Anatomy of the eustachian tube. Arch Otolaryngol. 1973, 97:2-8.
36. Paradise JL, Bluestone CD. Early treatment of the universal otitis media of infants with cleft palate. Pediatrics. 1974, 53:48-54.
37. Too Chung, MA. The assesment of middle ear function and hearing by tympanometry in children before and after palate repair. Br J Plast Surg. 1983, 36:295.
38. Smith TL, Diruggiero DC, Jones KR. Recovery of Eutachian tube function and hearing outcome in patients with cleft palate. Otolarngol Head Neck Surg. 1994, 111:423-29.
39. Kruse LS. Repercussões do momento da palatoplastia na otoscopia e audiometria de pacientes com fissura palatina entre seis e 12 anos de idade. Porto Alegre, 2005, (Dissertação de Mestrado - Universidade Federal do Rio Grande do Sul, Programa de Pós-Graduação em Medicina: Cirurgia).
40. Hassam ME, Askar S. Does palatal muscle reconstruction affect the functional outcome of cleft palate suregery? Plast Reconstruc Surg. 2007, 119(6):1859-65.
41. Kriens O. Anatomy of the velopharyngeal area in cleft palate. Clin Plast Surg. 1975, 2:261-83.
42. Fara M, Dvork J. Abnormal anatomy of the muscles of palatopharyngeal closure in cleft palate. Plast Reconstr Surg. 1970, 46:488-97.
43. Dickson DR. Anatomy of the normal and cleft palate eustachian tube. Ann Otol Rhinol Laryngol. 1976, 85(suppl 25):25-9.
44. Shibahara Y, Sando I. Histopathologic study of eustachian tube in cleft palate patients. Ann Otol Rhinol Laryngol. 1988, 97:403-8.
45. Matsune S, Sando I, et al. Insertion of the tensor veli palatini muscle into the Eustachian tube cartilage in cleft palate cases. Ann Otol Rhinol Laryngol. 1991, 100:439-46.
46. Sehhati-Chafai-Leuwer S, Wenzel S, et al. Pathophysiology of the Eustachian tube - Relevant new aspects for the head and neck surgeon. J Cran Maxilofac Surg. 2006, 34:351-54.
47. Carvalhal LHS, Costa SS, Collares MVM, Kruse LS. Otologic findings in patients with cleft lip and palate or isoled cleft palate. Otolaryngol Head Neck Surg. 2004, 131(2):269-270.
48. Altmann EBC, Vaz ACN, De Farias Ramos ALN, De Paula MBSF, Khoury RBF, Marques RMF. Tratamento Fonoaudiológico. Em: Altmann, EBC. Fissuras Labiopalatinas. 4a. Ed. Barueri: Pró-fono; 2005, pp. 367-403.
49. Dworkin JP, Marunick MT, Krouse JH. Velopharyngeal Dysfunction: Speech Characteristics, Variable Etiologies, Evaluation Techniques, and Differential Treatments. Language, Speech, and Hearing Services in Schools. 2004, 35:333-352.
50. Tortora GJ, Grabowski SR. Princípios de Anatomia e Fisiologia. 9a. ed. Rio de Janeiro: Guanabara; 2002.1047p.
51. Camargo LOS, Rodrigues CM, Avelar JA. Oclusão velofaríngea em indivíduos submetidos à nasoendoscopia na clínica de educação para saúde (CEPS). Salusvita Bauru. 2001, 20(1):35-48.
52. Altmann EBC, Lederman H. Videofluoroscopia da deglutição e do Esfíncter Velo-Faríngico: Padronização do Exame. Jorn Pró-fono. 1990, 2(1).
53. Pegoraro- Krook MI, Dukta-Souza JCR, Magalhães LCT, Feniman MR. Intervenção Fonoaudiológica na fissura palatina. Em: Tratado de Fonoaudiologia. 1ª. ed. São Paulo: Roca; 2004, cap.35. pp.439-455.
54. Johns DF, Rohrich RJ, Awada M. Velopharyngeal incompetence: a guide for clinical evaluation. Plast Reconst Surg. 2003, 112(7):1890-1897.
55. Trindade IEK, Trindade Junior AS. Avaliação funcional da inadequação velofaríngea. Em: Carreirão S, Lessa S, Zanini AS (eds). Tratamento das Fissuras labiopalatinas. 2.ed. Rio de Janeiro: Revinter; 1996, cap.26, pp. 223-225.
56. Whitehill TL, Chau CHF. Singleword intelligibility in speakers with repaired cleft palate. Clinical Linguistics & Phonetics. 2004, 18(4-5):341-355.
57. Altmann EBC, Ramos ALNFR, Khoury RBF. Avaliação Fonouadiológica. Em: Altmann, EBC (eds). Fissuras labiopalatinas. 2ed. São Paulo: Pró-fono; 2005, pp. 325-366.
58. Hanayama EM, Pinho SMRP, Tsuji DH. Ressonância nasal. Em: Pinho SMR. Tópicos em Voz. Rio de Janeiro: Guanabara Koogan; 2001, pp. 53-64.
59. Scherer, N.J. The Speech and Language status of toddlers with cleft lip and/or palate following early vocabulary intervention. Am J Speech-Language Pathol. 1999, 8:81-93.
60. Piccoli, EMH. Fissura Lábio-palatina: Considerações na Prática Clínica. Em: Pinho, SMR. Fundamentos em Fonoaudiologia: Tratando os distúrbios da Voz. Rio de Janeiro: Guanabara Koogan; 1998, cap.7, pp. 89-98.
1. Otorhinolaryngologist medical doctor. In course for Master's Degree in the Program for Post-Graduation in Medicine: Surgery of Universidade Federal do Rio Grande do Sul.
2. Phonoaudiologist. In Course for Doctor's Degree of Post-Graduation in Medical Sciences: Paediatrics of Universidade Federal do Rio Grande do Sul.
3. Phonoaudiologist. In Course for Master's Degree of Post-Graduation in Medical Sciences: Paediatrics of Universidade Federal do Rio Grande do Sul.
4. Otorhinolaryngologist Medical Doctor. Associate Professor of Otorhinolaryngology Discipline of the Medicine College of Universidade Federal do Rio Grande do Sul.
5. Doctor, Plastic Surgeon. Assistant Professor of Plastic Surgery Discipline of the Medicine College of Universidade Federal do Rio Grande do Sul. Head of the Craniomaxillofacial Surgery Service of Hospital de Clínicas de Porto Alegre.
Institution: Universidade Federal do Rio Grande do Sul, Hospital de Clínicas de Porto Alegre, Services of Otorhinolaryngology and Craniomaxillofacial Surgery, Otorhinolaryngology and Cleft Palate Ambulatory Porto Alegre / RS - Brazil.
Mail address:
Daniela Preto da Silva
Avenida Soledade, 569 - Conj. 805 e 806 - Torre Beta
Porto Alegre / RS - Brazil - Zipcode: 90470-340
Phone: (+55 51) 3378-9997
E-mail: danielapreto@hotmail.com
Article received on December 04, 2007.
Article approved on August 23, 2008.