INTRODUCTIONVascularization of the palate is carried by branches of the external carotid artery: greater palatine artery, ascending palatine artery and branches of the ascending pharyngeal artery (1,2).
The main artery that supplies the mucoperiosteum the hard palate is the greater palatine artery, which is a branch of the maxillary artery in the pterygopalatine fossa (3). The descending palatine artery passing through the greater palatine foramen and receives the name of greater palatine artery. This branch reaches the mucoperiosteum the hard palate and runs anteriorly, parallel to the lateral border of the hard palate, near its junction with the alveoli. Supplies the hard palate through the medial arch and alveolar bone via side branches (4). There is a large network of anastomoses between the vessels that supply the hard palate and soft palate.
Detailed knowledge of vascular anatomy of the palate and, in particular in the greater palatine foramen is important for prevention of vascular lesions during procedures in this region. During surgery to correct cleft palate is a common approach to the vascular pedicle for the release of the flap. Pedicle injuries may have catastrophic consequences such as necrosis of the flap. Moreover, it can be used in the manufacture of a palatal mucoperiosteal flap that can be introduced into the nasal cavity to the reconstruction of flaws in the skull base reconstruction after expanded endonasal approaches (5, 6).
The objective of this study is to develop an anatomical model that can illustrate the endoscopic anatomy of the greater palatine foramen and to determine whether injection technique is enough to fill the smaller arterial branches that supply the palate.
METHODVascularization of the palate is carried by branches of the external carotid artery: greater palatine artery, ascending palatine artery and branches of the ascending pharyngeal artery (1,2).
This study was developed at the Laboratory of Human Topographical Anatomy, School of Medicine, University of São Paulo. The project was approved by the Ethics Committee in Research of our institution. Five cadaver heads were prepared for dissection after intravascular injection of colored silicone (7), and stored in alcohol solution to 75%. Initially, both stumps of the cervical internal carotid artery and internal jugular veins were dissected and isolated. All vessels were cannulated with a catheter of appropriate diameter to completely fill the vascular lumen. After cannulation of the vessels, catheters were securely tied with cotton thread number 0. Importantly, this amarria should be firm to prevent leakage of silicone for injection. The vessels were repeatedly irrigated with water. Using a syringe of 60 mL was injected with water in the vascular system on one side to obtain a continuous flow of clear water on the contralateral side. For example, to repeatedly inject water into the common carotid artery on the right, expected to clean the system until the flow of water coming out of the left common carotid artery was clear and without clots. The same procedure was done to the venous system. The proper conduct of this step during the preparation of parts of corpses is essential for optimal results of staining of vessels. The presence of intraluminal clot is the main factor responsible for the failure of staining of vessels as the blood prevent the arrival of silicon, especially in smaller arterial branches and distal as in the case of the descending palatine (3). For staining of the arterial system, was used soluble red dye. For the venous system, we used water-soluble blue dye (Figure 1). We follow the following formulation for the preparation of silicone:
A) Arteries: Two pieces of polimetilsilaxane (thinner) for a piece of silicon.
B) Veins: A part of polimetilsilaxane (thinner) for a piece of silicon.
The catalyst (dialurato calcium carbonate) was added immediately before the injection in the vessels at the rate of 10 mL per 300 mL of solution. The dye (water-soluble pigments) was also added immediately before injection. During the endoscopic dissection of the palate, photographs were taken with a Nikon D70® digital camera 6.5-megapixel camera (Nikon, Tokyo, Japan) coupled to the endoscope. Digital images were stored on computer using the Windows Pictures Viewer® (Microsoft Corp., Redmond, WA).
Dissection Technique
Initially a autostatic retractor was positioned between the upper and lower lip. Due to the rigor mortis, only a minimum opening of the mouth was established. Opening this enough for the endoscopic dissection.
All the hard palate mucosa was inspected endoscopically with the identification of the transition region between hard and soft palate, and the transition between the mucosa attached to the alveolar bone and hard palate. An incision in inverted "U" was performed, taking as limits after the transition between hard and soft palate. The incision was always started on the right side, extending the earlier in the transition between the alveolar bone and the palatine bone (more later) and jawbone (anteriorly). Similar incision was repeated on the left. The anterior ends of the incisions were united before, giving the shape of inverted U (Figure 2). As a result, a subperiosteal detachment was performed to identify the major palatine foramen on both sides. The posterior limit of the detachment was the identification of the posterior border of the palatine bones and muscles of the soft palate.
RESULTSFive palates were dissected, making a total of 10 major palatine arteries evaluated. In 3 palates, the greater palatine artery was stained on both sides. In the other 2 cases, only the artery of one side was stained. In one such case, the artery on the right was stained, while the dye did not reach the artery on the left side (Figure 3). While in another case, the situation was the opposite. The artery was stained only the right side.
Thus, the total of 10 dissected arteries, 8 were properly stained injection technique used. This corresponds to an efficiency of 80%.
DISCUSSIONThe descending palatine artery arises from the pterygopalatine fossa (3) and continues downwards into the pterygopalatine canal (5). As the pterygopalatine canal close to the palate, its bony walls become slightly thickened, ending in greater palatine foramen. On the way out of this foramen, the vase is called major palatine artery and walks in the lateral portion of the palate, sending small branches to its anastomosis with branches of the sphenopalatine foramen incisive in (4).
The palatal flaps are based on the greater palatine artery, and the connection between the two major palatine arteries through the midline (8) allows the flap is based on only one major palatine artery (9), which greatly increases the potential size same. The flap can provide approximately 10 cm2 of tissue (5, 10), and can rotate 180o, be reversed or transposed into the nasal cavity. The versatility of this flap allows it to be used to correct flaws in the hard palate, soft palate, retromolar (10) and base (5.6). According to Olivier et al, after dissection of the pterygopalatine canal and transposition of the flap to the nasal cavity by extending the greater palatine foramen, it enabled the coverage of flaws in the plan dural sinus, sella and clivus to the level of the foramen magnum (5 ).
Shows how great advantage the fact that its production is not technically difficult (10), however, the main limitation of this flap is to hold and / or voltage level of the pedicle foramen greater palatine, which can cause vascular compromise with subsequent dehiscence, necrosis (10) or atrophy of the flap (4). The denuded palatal bone heals by secondary intention, but the mucosa response almost identical to normal tissue, which over time is also a process of reinnervation.
The development of anatomical models is very important in the production of educational materials, development of anatomical knowledge, in improving the ability to perform specific techniques, shortening the learning curve (1-4, 6, 7). The injection technique allows the color and precise anatomical dissection of vessels larger than 0.1 mm in diameter (2) and starting from the assumption that the descending palatine artery shows 2 mm gauge the level of the foramen greater palatine (1), all vessels studied should have been stained. However, presence of intraluminal clot may have prevented the arrival of silicone terminal arterial branches.

Figure 1. (A) Injection of colored silicone in red in the carotid system. (B) After injection of silicone in the arterial and venous (internal jugular vein) catheters are occluded with clamps hold.
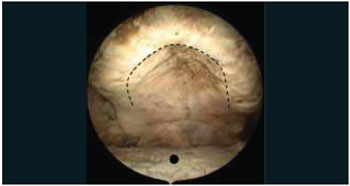
Figure 2. Photo intra-oral endoscope with the degree. Dotted line illustrates the region of the incision in the hard palate. Note that the incision is made in the mucosa of transition between the alveolar bone and the palatine bones. In this case, edentulous cadaver shows significant reduction in the amount of alveolar bone. Black circle shows the dorsal surface of the tongue.
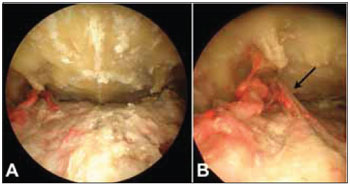
Figure 3. Photography The degree endoscope. After subperiosteal detachment of the flap of the palate, identifies the region of the greater palatine foramen on both sides. (A) Note that the right side, the artery was stained in red, while the left side, the silicone did not fill the pot. (B) Detail of the region of greater palatine foramen on the right. Note the greater palatine nerve exiting the foramen laterally to the arterial branches (arrow).
The anatomical model proved to be a reliable method for the endoscopic study of the greater palatine foramen, and the injection of silicone efficient in staining vessels terminals in 80% of cases
BIBLIOGRAPHICAL REFERENCES1. Vacher C. Vascularization of the palate in maxillary osteotomies: anatomical study. Surg Radiol Anat. 2002, 24(1):13-17.
2. Siebert, JW, Angrigiani C, McCarthy, JG, Longaker MT. Blood Supply of the Le Fort I Maxillary Segment: An Anatomic Study. Plast Reconstruc Surg. 1997, 100(4) Suppl 1:843-850.
3. Fortes FS, Sennes LU, Carrau RL, Brito R, Ribas GC, Yasuda A, Rodrigues AJ Jr, Snyderman CH, Kassam AB. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008, 118(1):44-49.
4. Huang MH, Lee ST, Rajendran K. Clinical implications of the velopharyngeal blood supply: a fresh cadaveric study. Plast Reconstr Surg. 1998, 102(3):655-667.
5. Oliver CL, Hackman TG, Carrau RL, Snyderman CH, Kassam AB, Prevedello DM, Gardner P. Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope. 2008, 118(12):2102-2106.
6. Hackman T, Chicoine MR, Uppaluri R. Novel Application of the Palatal Island Flap for Endoscopic Skull Base Reconstruction. Laryngoscope. 2009, 119(8):1463-1466.
7. Brito RV, Bento RF, Yasuda A, Ribas GC, Rodrigues AJ. Anatomy of the lateral base of the skull: development of a method of study. Int Arch Otorrinolaringol. 2005, 9(1):11-17.
8. Maher WP. Distribution of palatal and other arteries in cleft and non-cleft human palates. Cleft Palate J. 1977, 14:1-2.
9. Gullane PJ, Arena S. Extended palatal island mucoperiosteal flap. Arch Otolaryngol Head Neck Surg. 1985, 111:330-332.
10. Ward BB. The palatal flap. Oral Maxillofacial Surg Clin N Am. 2003, 15:467-473.
1. Specialist in Otorhinolaryngology ABORL-CCF. Graduate PhD in Otorhinolaryngology by FMUSP.
2. Specialist in Otorhinolaryngology ABORL-CCF. Otolaryngologist the Office of Medical Assistance to the State Civil Servants - SP (IAMSPE).
3. Lecturer, Discipline of Otorhinolaryngology, FMUSP. Associate Professor of Otorhinolaryngology at FMUSP.
4. Associate Professor, FMUSP. Chief of Surgery Craniomaxillofacial, HC-FMUSP.
Instituition: Faculdade de Medicina da USP. São Paulo / SP - Brazil. Mail Address: Prof. Dr. Luiz Ubirajara Sennes - 483, Teodoro Sampaio, St. - Pinheiros - São Paulo / SP - Brazil - ZIP. CODE: 05405-000 - Telephone: (+55 11) 3068-9855 - E-mail: lusennes@terra.com.br. Article received on March 9, 2010. Article approved on March 10, 2010.