INTRODUCTION The sudden sensorineural hearing loss (SSHL) is defined as auditory loss (AL) neuro-sensorial, equal or higher than 30 decibel (dB), in 3 or more consecutive frequencies, installed in a period of up to 3 days(1). Most of the times it is severe, not floating, unilateral and idiopathic(1, 2). In about one third of the cases can occur associated complaints as tinnitus, vertigo or dizziness and aural fullness(2, 3). Its incidence is from 5 the 20 cases per 100.000 inhabitants/year in the United States and new 15000 cases/year in the world, what represents 1% of all approximately the cases of SSHL(1, 4).
Since it was described for the first time, 62 years ago(5), innumerable etiologies have been suggested: infeccious(6), traumatic, neoplasia, immunological, ototoxic, vascular or ischemic, neurological, amongst others. Amongst the factors which are associated we have the metabolic disorders that are more frequent between cochlea -vestibulopath patients than in the general population(7). However, in only 10 to 15% of the cases the cause can be determined(1).
The treatment is controversial and can include, among others, antiinflammatory (steroids), vasodilatives, anti-viral, volumetric or hemodilutor, diuretic, antagonists of the calcium canals, hyperbaric chamber, carbogenic, anticoagulative expanders and recently corticosteroid intra-tympanic(8-13).
Approximately one third of the patients presents spontaneous improvement, most of them in first the two weeks of evolution of the disease(1, 3). The risk factors associated to a worse prognostic include: time of evolution (delay for beginning of the treatment), extremes of age, degree of the initial SSHL (severe losses), presence of initial associate vestibular symptoms, type of the tonal audiometric curve (descendent)(1, 4, 14).
The objective of this work is to observe the evolution of the SSHL in patients enclosed in different groups of treatment, according to the time of evolution of the illness and with the contraindications for use of medications.
CASUISTRY AND METHODOLOGY The design of the study configures one retrospect group of the handbook analysis of patients taken care in the Clinic of SS and enclosed in protocol of standardized treatment from January 1996 to January 2006. The effective ethical norms in the Institution, as determination of the Committee of Ethics and Research, have all been followed.
Our sample was constituted by 139 patients, with average age of 45.4 + 15.8 years (minimum = 13 years; maximum = 82 years), being 73 (52.5%) female and 66 (47.5%) of the male.
To be included in the study, the individual would have to present equal or bigger neuro-sensorial SSHL than 30 dB, in at least three consecutive frequencies, with sudden installation or in the maximum in 72 hours.
The patients were included in five groups of treatment, according to the time of beginning of the SSHL and the clinical conditions that qualified him/her, or not, to receive proposed medications . The following criteria have been used:
GROUP I: patients with installed history of SSHL for 5 days at most. They were submitted to the hospitalization and they received plasmatic expander (DEXTRAN
® 40000 UI, EV, every 12 h, up to 10 days), dexamethasone (8 mg, VO, once a day, 10 days, with project of gradual follow-up withdrawal), to acyclovir (200 mg, VO, 8/8 h, 15 days), acid nicotinic acid and hydrocloride of papaverine (30 mg and 100 mg, VO, every 12 h, 30 days), vitamin (50000 UI, VO, every 12 h, 30 days).
GROUP II: patients with history of SSHL installed from 0 to 15 days. Those who had clinical contraindication to the use of the plasmatic expander (systemic arterial hypertension, cardiopathy, coagulation disorder and renal insufficiency) were enclosed in this group. The therapeutical design of group I or III was prescribed, in the dependence of the time of beginning of the SSHL, however without the plasmatic expander.
GROUP III: patients with history of SSHL installed from 6 up to 15 days. It received the therapeutical design from group I, however without acyclovir.
GROUP IV: patients with history of SSHL installed from 16 up to 30 days. It received the therapeutical design from group I, however without plasmatic expander and acyclovir.
GROUP V: patients with installed history of SSHL for more than 30 days. It received nicotinic acid and papaverine hydrochloride (30 mg and 100 mg, VO, every 12 h, 30 days) and vitamin (50000 UI, VO, every 12 h, 30 days).
Audiometric serial examinations were carried through in all patients, before (initial) and after the beginning of the therapy (30, 90, 120 and 180 days).
The patients of groups I and III were hospitalized, as protocol above described, in order to receive plasmatic expander through endovenous way. In these cases, the tonal audiometry threshold was carried through on alternated days, the suspension of medication and the hospital discharge being programmed in the absence of improvement of the auditory thresholds after three days. The recovery of at least 10dB in 3 consecutive frequencies or of 15dB in two consecutive frequencies or 3 20dB in an isolated frequency in the tonal audiometry or still 3 15% in the speech recognition rate (SRR) were considered criteria of audiometric improvement. In the cases of audiometric improvement, the therapeutical design was kept by up to 10 days.
For documentation of the audiometric variation the PTA was used, pure tone average or pure tonal threshold. The PTA for low pitch frequencies was determined by the average of the tonal thresholds of the frequencies of 250, 500 and 1000 Hertz (Hz) and the PTA for high pitch frequencies from the average of the 2000 frequencies, 4000 and 8000 Hz. The evolution or audiometric response of the patients among the several groups of treatment was determined by subtracting the initial PTA (first day of medical evaluation) from the end (after 180 days).
For analysis statistics of the results, test t of Student and the analysis of variance were used (ANOVA). The considered level of significance was of p<0.05 in bicaudal tests.
RESULTS In 129 (92.8%) patients the SSHL was unilateral and in only 10 (7.2%) it was bilateral. In terms of ethnics 77 (55.4%) were white, 35 (25.2%) were black and 4 (2.9%) were of the yellow race. In 23 (16.5%) cases this data were not computed in handbooks. The time of evolution of the SSHL until the first examination was in average 17.2 + 24.6 days (minimum = 0 day; maximum = 120 days).
The groups of treatment revealed similar in terms of the averages of the initial PTA, both for low pitch frequencies and for high pitch ones (Table 1). Considering all the assisted cases, a significant reduction in the average of the PTA was observed after the treatment when compared with the initial values, both for low pitch frequencies and for high pitch ones(Table 2).
With relation to the comparison it among the groups in terms of the evolution or audiometric response to the treatment, determined by the difference between final and initial PTA, both for low pitch frequencies and for high pitch ones, there was no statistical significance (Table 3).
We observe that a significant inverse linear correlation exists between the time passed until the first examination and the evolution or audiometric response (initial PTA - final PTA) of the patient to the treatment. Therefore, the bigger the time until the beginning of the treatment, the lesser the audiometric improvement. Thus, for the low pitch frequencies we observe a coefficient of correlation of Spearman r = - 0.45 (p < 0,001; IC 95% = - 0.58 to - 0.29) (Graph 1) and for high pitch frequencies r = - 0.42 (p < 0.001; IC 95% = - 0.55 to - 0.25) (Graph 2).
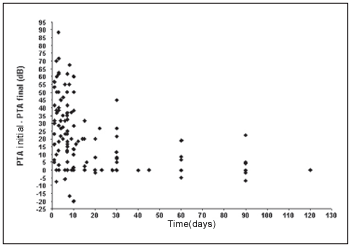
Graph 1. Correlation between the time of beginning of the treatment and the evolution in the low pitch frequencies.
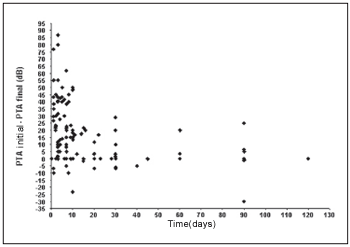
Graph 2. Correlation between the time of beginning of the treatment and the evolution in the low pitch frequencies.
Literature shows that the average age of the patients with SSHL varies from 43 to 53 years, that the distribution for sex is equivalent and that the onset in more 95% of the cases is unilateral, similar to what we have observed in this survey(2, 4, 13, 15, 16). The only epidemiologist survey of SSHL published until the moment was made in Japan in three distinct years, in different decades and shows that the time between the beginning of the SSHL and the first examination varied from 11.6 ± 12.1 days in 1974, 9.1 ± 9.8 days in 1987 to 8.1 ± 9.1 days in 1993(15). We cannot directly compare our casuistry with the one by Nakashima et al(15), for the type of design and being different populations, however we observe delay until the first examination what can be attributed in part to the indifference of the patients in relation to the symptom or the difficulty of access to the doctor.
Based on homogeneous groups in terms of the initial averages of the PTA and considering all the assisted cases, there was significant reduction in the average of the PTA. This average of improvement (initial PTA - final) was of 21.6dB for low pitch frequencies and 16.7dB for the high pitch. Although there was no control group for comparison, literature shows that the spontaneous improvement (without treatment) occurs in 32% of the SSHL cases and is in average of 15dB of PTA(3, 8), therefore lower than the one observed in this study. The estimated audiometric recovery with the treatment is of approximately 25dB of PTA(3), similar to the one that we observe for low pitch frequencies.
In terms of the evolution of the patients (initial PTA - PTA final) in the five groups of treatment, the observed difference was not significant, both for low pitch frequencies and for high pitch ones. Although we have not observed statistical significance, the improvement of the PTA was lower in the patients of groups IV and V, who initiated the treatment 15 days after the beginning of the SSHL. Groups I, II and III initiated the treatment in first the 15 days of SS, however the best PTA was observed in group II, the only one which did not receive plasmatic expander for clinical contraindication. Although some base illnesses are capable to intervene with the microcirculation of the internal ear making the clinical improvement of the SSHL difficult, the comorbities presented for the patients of group II, did not apparently mean factors of worse prognostic. In our survey, the plasmatic expander did not contribute to potentiate the effect of the corticosteroid and of acyclovir in the treatment of the SSHL, once the group that better answered to the therapy did not used it.
We observe a significant inverse linear correlation between the passed time until the beginning of the treatment and the audiometric response presented by the patient, both for low pitch frequencies and for high pitch. We agree, therefore, with reports by other authors who show that the prognostic is better as much as precociously the treatment is started(1, 4, 17).
CONCLUSION After our survey, we can infer that:
- The plasmatic expander did not improve the efficiency of the association between corticoid and acyclovir for the SSHL treatment;
- The precocious beginning of the treatment determines the best disease evolution.
BIBLIOGRAPHY1. Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngol Clin North Am 1996;29(3):393-405.
2. Psifidis AD, Psillas GK, Daniilidis J. Sudden sensorineural hearing loss: long-term follow-up results. Otolaryngol Head Neck Surg 2006;134(5):809-15.
3. Chen CY, Halpin C, Rauch SD. Oral steroid treatment of sudden sensorineural hearing loss: a ten year retrospective analysis. Otol Neurotol 2003;24(5):728-33.
4. Byl FM, Jr. Sudden hearing loss: eight years experience and suggested prognostic table. Laryngoscope 1984;94(5 Pt 1):647-61.
5. DeKleyn A. Sudden complete or partial loss of function of the octavus system in apparently normal persons. Acta Otolaryngol (Stockh) 1944;32:407-429.
6. Lorenzi MC, Bittar RS, Pedalini ME, Zerati F, Yoshinari NH, Bento RF. Sudden deafness and Lyme disease. Laryngoscope 2003;113(2):312-5.
7. Bittar R, Bottino M, Zerati F, Moraes C, Cunha A, Bento R. Prevalency of metabolic disorders in dizzy patients. Rev Bras Otorrinolaringol 2003;69(1):64-68.
8. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A doubleblind clinical study. Arch Otolaryngol 1980;106(12):772-6.
9. Cinamon U, Bendet E, Kronenberg J. Steroids, carbogen or placebo for sudden hearing loss: a prospective double-blind study. Eur Arch Otorhinolaryngol 2001;258(9):477-80.
10. Probst R, Tschopp K, Ludin E, Kellerhals B, Podvinec M, Pfaltz CR. A randomized, double-blind, placebo-controlled study of dextran/pentoxifylline medication in acute acoustic trauma and sudden hearing loss. Acta Otolaryngol 1992;112(3):435-43.
11. Stokroos RJ, Albers FW, Tenvergert EM. Antiviral treatment of idiopathic sudden sensorineural hearing loss: a prospective, randomized, double-blind clinical trial. Acta Otolaryngol 1998;118(4):488-95.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, Wit HP, Albers FW. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol 2003;112(11):993-1000.
13. Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am 2004;37(5):1061-74.
14. Harada H, Kato T. Prognosis for sudden sensorineural hearing loss: a retrospective study using logistical regression analysis. Int Tinnitus J 2005;11(2):115-8.
15. Nakashima T, Itoh A, Misawa H, Ohno Y. Clinicoepidemiologic features of sudden deafness diagnosed and treated at university hospitals in Japan. Otolaryngol Head Neck Surg 2000;123(5):593-7.
16. Fetterman BL, Luxford WM, Saunders JE. Sudden bilateral sensorineural hearing loss. Laryngoscope 1996; 106(11):1347-50.
17. Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 1977;86(4 Pt 1):463-80.
1. Medicine PhD (Assistant Doctor of the Otoneurology Sector at the FMUSP Otorrhinolaryngology Department)
2. Otorrhinolaryngologist Medical Doctor (Otorrhinolaryngologist of the SSHL Clinica at HCFMUSP)
3. Otorrhinolaryngologist Medical Doctor (PhD Student of the FMUSP Otorrhinolaryngology Department)
4. Otorrhinolaryngologist Medical PhD Doctor (Assistant Professor of the FMUSP Otorrhinolaryngology Department)
5. Otorrhinolaryngology Professor (Assistant Professor of the FMUSP Otorrhinolaryngology Department)
Departamento de Otorrinolaringologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Otorrhinolaryngology Department at Clinical Hospital of São Paulo University Medicine School) (HC-FMUSP)
Roseli Saraiva Moreira Bittar
Address: Av Dr. Enéas de Carvalho Aguiar, 255 - 6º andar / sala 6167 - São Paulo/SP - CEP: 05403-000 - Telefone/Fax: (11) - 3727.2873 - E-mail: otoneuro@hcnet.usp.br
This article was submitted to SGP (Publishing Management System) of R@io on June 20, 2007 and approved on August 8, 2007