 |
742 |
|
| Year: 2011 Vol. 15 Num. 1 - Jan/Mar - (9º)
|
|
 |
| Section:
Original Article
|
|
|
|
|
| Profile of Patients of the Auditory Health of the State of Santa Catarina Served at HU-UFSC |
|
| Author(s): |
| Oscar Cardoso Dimatos1, Cláudio Márcio Yudi Ikino2, Paulo Arlindo Philippi3, Spyros Cardoso Dimatos4, Marília Susane Birck1, Paulo Fontoura Freitas5.
|
|
|
| Key words: |
| hearing, neurosensorial hearing loss, hearing impaired rehabilitation. |
|
|
 |
| Abstract: |
Introduction: Hearing is one of the essential functions for the development of language and its deficiency may lead to several consequences. Objective: To describe the profile of patients of the Hearing Health Program of the State of Santa Catarina attended at the College Hospital of the Federal University of Santa Catarina. Method: We performed a retrospective study with the patients with hearing aid indication attended in the years 2007 and 2008. They were divided into 2 groups: children (< 18 years) and adult (> 18 years). We assessed the following: age, sex, loss type and degree, duration, co-morbidity and probable etiology. Results: 304 reports were reviewed, 10.2% of the children group and 80.8% of the adult group. In the children group, the mean age was of 7.7±5,4 years, with 58.06% boys and 41.94% girls, and in the adult group the mean age was of 61±16 years, with 52.38% women and 47.62% men. The neurosensorial hearing loss was found more frequently in both groups. In the children group, the severe degree loss was more frequent, and in the adult group, the moderate and moderate to severe degrees were more frequent. The main etiologies were genetic/unknown causes in the children group and presbycusis in the adults group. Conclusion: In the children group, most was formed by boys with neurosensorial loss of light and severe degrees, duration > 1 year and without co-morbidities. In the adult group, most was formed by women with neurosensorial loss of moderate and moderate-severe degrees, duration > 5 year and with co-morbidities.
|
|
 |
INTRODUCTION
According to the World Health Organization (WHO), hearing loss is the most frequent sensorial deficit in the human population that affects over 250 million people in the world (1). The Brazilian Institute of Geography and Statistics (IBGE) confirms that 3.37% of the Brazilian population (estimated by the 2000 census in 169.872.856 people) have hearing loss. Data published by IBGE 2000 describes that 0.8% (406.588) of the Brazilians aged up to 14 years suffer from hearing loss, while 2.95% (3.240.263) of Brazilians aged between 15 and 64 years old, and 21% (2.088.247) of Brazilians aged 65 ears or older have hearing loss. In the population with hearing loss and aged up to 14 years, 54.5% are men; in the group aged between 15 and 64 years, the proportion is of 54.2%; and 49.75% in the group of 65 years or older (2).
Hearing is one of the most important functions for development of language and consequently for communication. However, the impact caused by hearing loss has been taken for granted by the society, government and health professionals (3). The hearing loss leads to several consequences, such as difficulty to assimilate discourse, reduction of the capacity of communication, delaying of the language acquisition in children, social isolation, educational and economical deficit, and stigmatization (1, 3).
The hearing losses have several etiologies and may be classified as regards to type, degree and age of affection. As regards to type, they may be conductive, neurosensorial or mixed. The degree may be light, moderate, moderate-severe, severe, profound. The affection age may be prenatal, perinatal and postnatal (3, 4).
The prenatal causes act during pregnancy, affects the embryo and may have etiologies like congenital rubella, ototoxic medicines, genetic alterations, syphilis, toxoplasmosis and bacterial infections. The perinatal causes commit the newborn from the beginning of the birth until about 8 days after it and the main examples include prolonged birth, prematurity, anoxia and kernicterus. The postnatal causes are acquired along development. They may affect the external ear, such as cerumen, infections and traumatisms. The alterations of the middle ear may be: traumatisms, tubal obstruction, otitis media. When there are damages to the inner ear, the hearing loss is called neurosensorial and, amongst the main etiologies, infections, ototoxic drugs and tumors are included (4). At adult age, the importance of diagnosis of diseases such as presbycusis and noise-induced hearing loss is critical (5).
Due to the high prevalence of dysacusis and the harmful consequences it may cause, the prevention plays a crucial role for reduction of this problem (5). Additionally, the prevention of deafness is evidently less expensive than its treatment (prosthetization or cochlear implant) (6). Aiming to enhance prevention programs and diagnosis of hearing loss, this study carried out a 2-year analysis for execution of the Hearing Health Program of Santa Catarina, in order to determine the profile of the patient attended at the College Hospital of the Federal University of Santa Catarina that is a reference in the high complexity of this program, is in charge of selection and fitting of hearing aids and compare children to adults hearing aid users. Therefore, we expect to contribute for improvement of the service for this type of patient.
METHOD
The design of this paper was approved by the Ethics Committee in Research with Human Beings from UFSC (design no. 336/08).
This research is formed in a transversal study. We selected at random patients forwarded for service at the Medical School of the Federal University of Santa Catarina, of the Hearing Health Program of the State of Santa Catarina, attended in the service of Otorhinolaryngology in the period from January 2007 through December 2008. The study includes patients with hearing loss and indication for hearing aid.
The patients were classified into two groups: pediatric, aged less than 18 years old, and adult, aged 18 years or older. They were evaluated according to age, sex, type of loss, degree of loss, duration according to four categories (as long as 1 year, equal or longer than 1 year and shorter than 5 years, equal or longer than 5 years and shorter than 10 years, equal or longer than 10 years), connected diseases and probable etiology. For evaluation of the type and degree of loss we collected data from both ears per patient and evaluated the tonal audiometric exams and/or encephalic trunk audition evoked potential (EAP).
In the classification of the degree of hearing loss, when the mean tonal thresholds of 500 to 3000 Hz were normal and there was hearing loss in some other frequency, we applied the tonal thresholds average of such frequencies altered for classification. For patients in whom the hearing evaluation was made only with EAP, we used the electrophysiological threshold for classification.
The audiometry was made with the equipment of models SIBELMED AC-50D, INTERACOUSTICS AC33 and AD 229e, in acoustic cabin, with the use of earplug and bone vibrator, by assessing the auditory thresholds by airway in the frequencies of 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. The osseous pathway was tested in the frequencies of 500, 1000, 2000, 3000 e 4000 Hz. The encephalic trunk audition evoked potential (EAP) was carried out with equipment Smart Box Jr., with the use of click type sound stimulus.
The statistical analysis was made with the help of two softwares: Statcalc in Epiinfo 6, used for computing of prevalence ratios and significance of the associations calculated by the chi-square test at the confidence level of 95% (p < 0.05) and EpiCalc 2000, used for computing the confidence intervals
RESULTS
304 patients' records were analyzed: 31 (10.2%) of the children group and 273 (89.8%) of the adults group. In the pediatric group, the ages ranged from 4 months to 16 years old, with a mean age of 7.7±5.4 years old. In the adults group, the ages ranged from 19 to 93 years, with mean age of 61±16 years old. The frequency per sex was distributed as follows: in the pediatric group, 18 (58.06%) of the patients of the male sex and 13 (41.94%) patients of the female sex were observed, while in the adult group 130 (47.62%) of the patients of the male sex and 143 (52.38%) of female sex were observed.
As regards to the type of loss, in the pediatric group 46 included neurosensorial losses (74.19%, CD95%):61,26-84,10), 3 mixed (4.83%, IC95%:1.26-14.38) and 1 conductive (1.61%, IC95%: 0,08-9,83). 2 cases were observed with normal hearing (3.22%, IC95%: 0.56-12.17) and in 10 times the data was not completed (16.12%, IC95%): 8.41-28.13). In the adult group 387 included neurosensorial losses (70.87%, IC95%: 66.84-74.62), 145 mixed (26.55%, IC95%: 22.94-30.51) and 13 conductive (2.38%, IC95%: 1.33-4.14). There was 1 case of normal hearing (0.18%, IC95%: 0,01-1,18). The statistical analysis results showed there was no significant difference as regards to the prevalence of the neurosensorial and conductive losses between both groups (p=0.58 and p=0.70, respectively). The major prevalence of mixed loss in the adult group was statistically confirmed (RP=5.49; p<0.001).
The distribution of the patients as for the degree of loss is described in Figures 1 and 2. The result of the statistical analysis showed there was no significant difference as for the prevalence of light and severe degrees losses between both groups (p=0.49 and p=0.68, respectively). The major prevalence was statistically confirmed of moderate degree losses (RP=3.13; p<0.001) and moderate-severe (RP=1.84; p=0.036) in the adult group and the major prevalence of profound degree loss in the children group (RP+4.24; p<0.001).
As regards to the duration of the loss, in the pediatric group there were 3 (9.57%) cases with a duration of hearing loss shorter than 1 year, 10 (32.25%) cases with duration equal or longer than 1 year and shorter than 5 years, 4 (12.90%) cases with loss duration equal or longer than 5 years and shorter than 10 years, 7 (22.58%) occurrences of duration equal or longer than 10 years, and in 7 (22.58%) patients, it was not possible to determine the duration of loss. In the adult group, there was 1 (0.36%) case with duration of hearing loss lower than 1 year, 46 (16.84%) cases with duration equal or longer than 1 year and shorter than 5 years, 39 (14.28%) patients with hearing loss duration equal or longer than 5 year and shorter than 10 years, 126 (46.15%) occurrences with duration equal or longer than 10 years, and in 61 (22.34%) cases the duration of the loss was not determined.
As far as the associate diseases are concerned, in the pediatric group there were 8 (22.22%) cases of neurological diseases and other 6 cases of comorbidity (nephropathy, rhinitis, cardiopathy, reduction of visual accuracy, hyperactivity and leukemia) with 1 (2.77%) occurrence for each of them, and in 22 (61.11%) cases, the patients did not present with any associate diseases. In the adult group, there were 105 (30.43%) occurrences of systemic arterial hypertension, 30 (8.69%) of diabetes mellitus, 27 (7.82%) cases of cardiopathy, 12 (3.47%) neurological diseases, 8 (2.31%) psychiatric disorders, 6 (1.73%) of dislypidemia, 6 (1.73%) of hypothyroidism, 5 (1.44%) of visual disability, 3 (0.86%) of pneumonias, 3 (0.86%) of benign prostatic hyperplasia, 2 (0.57%) of dermatological diseases, 2 (0.57%) of gastrointestinal diseases, 2 (0.57%) of cancer and 4 other associate diseases (labyrinthopathy, imperfect osteogenesis imperfecta, gout and locomotion problems) with 1 (0.28%) occurrence per disease, and in 130 (37.68%) cases no comorbidity was reported.
The distribution of patients as regards to the probable etiology of hearing loss is presented in Figures 3 and 4.
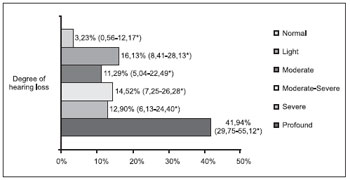 Figure 1. Degree of hearing loss of the pediatric group (n=62). * Confidence Interval of 95%.
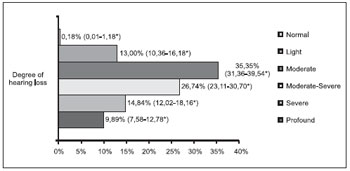 Figure 2. Degree of hearing loss of the adult group (n=546). * Confidence Interval of 95%.
DISCUSSION
In the studies by Nóbrega et al (7) for the period of 1994-2000, Dereköy (8), Kittrell e Arjmand (9) demonstrated that the children with hearing loss of the male sex prevailed on those of the female sex, respectively, at the proportion of 1.2:1, 1.54:1 and 1.27:1. In our study, in the pediatric group, the male sex prevailed on the female sex in the proportion of 1.38:1, according to the aforesaid studies.
In the study by Fortes et al. (5), the proportion of patients with hypacusis, taking into account all age ranges and the 277 patients attended (273 with hypacusis and 4 with peripheral facial paralysis), was similar, with a slight prevalence of men (1.06:1). In the study by Abdel-Hamid et al. (10), the proportion of men and women with hearing loss aged equal or older than 15 years was similar, with a slight prevalence of women (1.08:1). In our research, the proportion of women in the adult group was slightly higher than that of men (1.1:1).
through analysis of 131 reports of students attending a school for deaf students, Cecatto et al. (06) confirmed that the neurosensorial hearing loss was the most common, representing 99% of the cases. Lee et al. (11) evaluated 234 patients who procured hearing aids and out of the 468 ears analyzed 77.56% had neurosensorial hearing loss, and this type of loss is the most common for all age groups. Additionally, in our sample the neurosensorial hearing loss was the most common loss, representing 74.19% and 70.87% in the children and adults groups, respectively.
In the survey by Kittrell and Arjmand (9), who evaluated 291 hearing impaired aged between 4 and 20 years old, the hearing loss of profound degree represented 82% of the cases. In the study by Cecatto et al. (6), the loss of profound degree occurred in 65%. In our work, we proved statistically the higher prevalence of loss of profound degree in the children group.
In the study by Veras and Mattos (12), 69.6% of the elders with hearing loss had loss of light degree taking into account the best ear. Wilson et al. (13) estimated the prevalence of hearing loss in people aged 15 years or older and noted that, considering the best ear, the loss of light degree was also the most frequent. In our research, differently from the studies mentioned, the moderate and moderate-severe degrees prevailed in the adult group, probably because the patients had indication for the use of hearing aid, which means a stronger severity of the deficiency presented.
The early diagnosis of the hearing loss in children demands a detailed anamnesis (4). As well as in the protocol used in this study, issues about infections, use of ototoxic drugs, data regarding birth (prematurity, anoxia, hyperbilirubinemia), general deformities research and cases of hearing loss in the family (4) should be included in the history.
In many situations, even with a detailed investigation, the precise etiology of the hearing loss is not possible to define. In the studies by Cecatto et al. (6), Dereköy (8) and Walch et al. (14) no defined etiology was described, respectively 33 (25.2%) patients, 34 (26.1%) children and 47 (44%) children.
The high incidence of unknown etiology in the pediatric group in this research confirms the studies described and demonstrates the application of a standardized questionnaire failed to determine the etiology. The confirmation of the etiological diagnosis of hearing loss still composes a great challenge for most researchers (7). In our work, such difficulties in the identification of the etiology were also found in the adult group, since the unknown causes represented the second most common cause of hearing loss in this group.
The genetic causes of hearing loss are present in most studies (7). In the study by Nóbrega et al. (7), the genetic loss occurred in 15.73% of the patients in the period of 1990-1994, and in 8.33% of the children in 1994-2000. In the study by Dereköy (8), carried out in a school for deaf people, the genetic etiology represented the third most common cause of auditory deficiency, with 31 (23.8%) children affected, after fever convulsion, with 35 (26.9%), and unknown cause with 34 (26.1%). This study confirmed the unknown genetic etiology represented in 33.33% of the cases in the pediatric group was the most expressive cause of hearing loss, confirming the importance of both etiologies in this group.
The main perinatal causes resulting in hearing loss include anoxia, prematurity, fetal erythroblastosis and kernicterus (4). According to Streppel et al. (15), the accumulation of perinatal causes is the reason for most acquired etiologies of hearing loss in the German children. According to such authors, the advance of neonatology brought the diminishment of perinatal mortality and an increase of perinatal complications, such as acquired hearing loss.
In our studies, the perinatal causes represent the second most common cause of deafness in the pediatric group and such progress of pediatrics is a possible explanation for such findings. The statistical analysis showed there is no significant difference between the frequency of the genetic/unknown and the perinatal causes in the pediatric group. In the adult group, the genetic and perinatal causes were not common, which demonstrates the importance of other etiologies in this age range, such as presbycusis, PAIR, otosclerosis and COM.
Presbycusis, a hearing loss for aging, is indicated as the main cause of audition deficiency in older people according to the international literature, with the incidence of about 30% in the population aged more than 65 years old (16). According to Veras and Mattos (12), who performed a literature review on this theme, presbycusis has been considered to be the most common cause of hearing loss in the elderly people in Brazil, which implies a difficulty to understand during verbal communication. In our work, presbycusis was the main cause of hearing loss in the adult group, which is according to the abovementioned references. We confirmed statistically that presbycusis is the most frequent etiology in the adult group.
Noise induced hearing loss (NIHL) is the main cause of deafness and hearing loss in the United States. Although aging and genetics are the main risk factors, the temporary or permanent hearing loss is becoming increasingly common among young adults and children, especially with the increase of portable music devices exposure (17). Therefore, they are to be guided and informed to avoid the use of such equipment. In our study, no case of NIHL was found in the pediatric group, while in the adult group NIHL was responsible for 5.63% of the causes of hearing loss.
Otosclerosis is one of the most frequent causes of hearing loss in the adult individual (18). It is more frequent in the female sex, in the proportion of 2:1, and may bring a worsening of the symptoms during pregnancy (18, 19). Its incidence is more common between 20 and 40 years, and is less common in children and adults older than 50 years (18, 20). In our research, the incidence of otosclerosis was more frequent in women, in the proportion of 2:1, with 41.6% of the patients in the age range of 20 to 50 years, absent before 35 years old. It is possible that most cases after 50 years old are somehow justified by the indications of hearing aid for otospongiosis and for the period of time waiting for scheduling of consultation, since the Medical School is a state reference of the Auditory Health Program. One of the main indications for the use of hearing aid for otosclerosis is in those patients who cannot submit to surgical treatment, and most of whom are elderly. Additionally, in these population of elderly, otosclerosis connected to neurosensorial affection is more frequent, where surgery doesn't modify such loss, and the indication of hearing aid is more common.
In the study by FORTES et al. (5), the infectious etiology represented approximately 70% of the cases of hearing loss in the age range below 40 years old, which diminished with advancement of the age, but still representing 31% of the cases in the population older than 60 years, most cases of otitis media of the chronic (79%) and secreting (18%) types, and only 1.2% of acute otitis media. In a research with 4000 Egyptians, to estimate the prevalence of causes of hearing loss in Egypt, Abdel-Hamid et al. (10) confirmed that 16.02% (641) participants had hearing loss and the 3 most common causes were: otitis media with effusion (30.7%), presbycusis (22.7%) and chronic suppurative otitis media (13.2%).
In our research, COM, that was included in the sequel subgroup of otitis media in the pediatric group, occurred in 3 patients of this group and in 19 (5.94%) patients of the adult group, representing the third most frequent identifiable cause of hearing loss in this last group. It is possible that such cases of COM in our work, proportionally minor in the studies mentioned, have occurred for several reasons, amongst which the fact we analyze only patients with indication of hearing aid. This allows that the etiologies, whose treatment is mainly based on the use of hearing aid, such as presbycusis, stand out compared to the causes in which the clinical and surgical treatments, be effective for a large number of patients, like in the cases of chronic otitis media with effusion.
Acquired infections are other important causes of hearing loss. Among the acquired infections, the meningitis is doubtlessly the main one and indicated as the most relevant postnatal etiology of neurosensorial dysacusis (6, 15). In the studies of Cecatto (6) and Butugan (21), patients with age ranging from 3 to 30 years old and in the first year of life were evaluated, respectively, and the incidence of hearing loss for meningitis was of 8.4% in both works. This study attended 3 cases of meningitis in the pediatric group, representing, along with the chronic otitis media, the most common postnatal cause of auditory deficit in this age range.
According to Nóbrega et al. (7), congenital rubella is still one of the main etiologies of hearing loss in our field. Cecatto et al. (6) described the congenital rubella is the main prenatal cause of hearing loss for their teratogenic effects and demonstrated the congenital rubella corresponded to 31 (23.6%) of the cases and was the most common identifiable cause of hearing loss. During the period of 1990-1994, Nóbrega et al. (7) confirmed that the congenital rubella was the second most common cause of hearing loss in children and adolescents, only preceded by the unknown etiology.
In the study by Dereköy (8), who evaluated 130 deaf students in the age range of 5 to 16 years old in a school in Turkey, there was no case of maternal rubella as a cause of deafness. Likewise, there was no syndrome of congenital rubella in other 6 studies in Turkey. As this country did not apply a program of immunization against rubella yet, the natural immunity against rubella is high. According to Streppel et al. (15), the reduction of the cases of congenital rubella detected in Germany seems to be due to the success of vaccination.
In our study, the congenital rubella represented, in the pediatric group, the least common cause of hearing loss along with the use of ototoxic drugs, confirming a discrepancy with the national studies; but was according to the Turkish and German studies. In the studies performed by Nóbrega (7) and Cecatto (6), the patients were evaluated in the years 1990-2000, and in 2001, respectively. The vaccination actions of 2001-2002 of the Brazilian Ministry of Health having been directed generally to women aged from 12 to 39 years old led to a reduction of the incidence of rubella. However, this vaccination coverage was not homogeneous, and accumulated susceptible agents and contributed to outbreaks in 2006 and 2007 (22).
Such outbreaks didn't reach large proportions in Santa Catarina that had 92 cases confirmed of rubella in the years 2006 and 2007, compared to other states, such as Rio Grande do Sul, that had 2866 cases in the same period (23, 24). Aiming to interrupt the circulation of the rubella virus in Brazil, the Ministry of Health promoted the vaccination campaign of 2008 for men and women, intended for age range of 20-39 years old, that was defined as susceptible population (22). The tendency is that in the following years there is a significant fall of cases of rubella in the country and consequently of congenital rubella syndrome.
As regards to the ototoxicity in the childhood, this includes newborns with severe infection in the nursery of intensive care who receive aminosidine antibiotics. Several authors have published variable percentages of cases of ototoxicosis in children, resulting from the use of several aminosidine antibiotics. Other authors report the absence of ototoxicosis in children who received such drugs (3). In our research, 3 (5.88%) cases of hearing loss were attended in the pediatric group, having as a probable etiology the use of ototoxic drugs, and in 2 children gentamicin was applied.
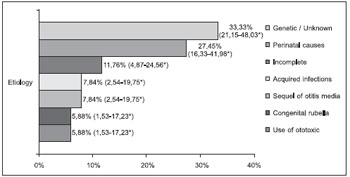 Figure 3. Probable etiology in the pediatric group (n=51). * Confidence Interval of 95%.
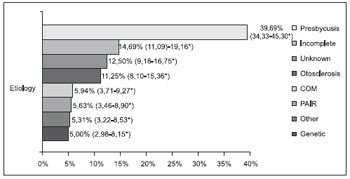 Figure 4. Probable etiology in the adult group (n=320). * Confidence Interval of 95%.
CONCLUSION
Most patients of the pediatric group forwarded for service in the Medical School of the UFSC, of the Auditory Health Program of the State of Santa Catarina, are of the male sex, with mean age of 7.7 years old, and with neurosensorial hearing loss of light and profound degrees, with duration of loss equal or longer than 1 year and without associate diseases. The genetic/unknown etiologies and the perinatal causes represented over 60% of all causes in this group. Most patients of the adult group sent for service are of the female sex, aged on average 61 years old, with neurosensorial hearing loss, of moderate and moderate-light degrees, with duration of loss equal or longer than 5 years, and the systemic arterial hypertension is the most frequent associated disease. Presbycusis was the most common etiology in the adult group.
The comparison of both groups shows the prevalence of hearing loss of the mixed type in the adult group. As for the degree, there was a major prevalence of losses of moderate and moderate-severe degrees in the adult group and profound degree in the pediatric group. The main identifiable etiologies were different in both groups.
BIBLIOGRAPHICAL REFERENCES
1. World Health Organization [homepage na Internet]. Mathers C, Smith A, Concha M. Global Burden of hearing loss in the year 2000 [acesso em 2009 Out 29]. Disponível em: www.who.int/entity/healthinfo/statistics/bod_hearingloss.pdf
2. Instituto Brasileiro de Geografia e Estatística [homepage na Internet]. Censo de 2000 [acesso em 2009 Out 29]. Disponível em: http://www.ibge.gov.br/home/estatistica/populacao/censo2000/populacao/censo2000_populacao.pdf
3. Costa SS, Cruz OLM, Oliveira JAA. Otorrinolaringologia: Princípios e Prática. 2ª ed. Porto Alegre: Artmed, 2006.
4. Costa SS, Cruz OLM, Oliveira JAA. Otorrinolaringologia: Princípios e Prática. 1ª ed. Porto Alegre: Artes Médicas, 1994.
5. Fortes FSG, Franceso RC, Bento RF, Miniti A. Liga de Prevenção à Surdez: Análise de Três Anos de Atuação. Arq Int Otorrinolaringol. 2002 , 6(4):302-9.
6. Cecatto SB, Garcia RID, Costa KS, Abdo TRT, Rezende CEB, Rapoport PB. Análise das principais etiologias de deficiência auditiva em Escola Especial "Anne Sullivan". Rev Bras Otorrinolaringol. 2003, 69(2):235-40.
7. Nóbrega M, Weckx LLM, Juliano Y. Study of the hearing loss in children and adolescents, comparing the periods of 1990-1994 and 1994-2000. Int J Pediatr Otorhinolaryngol. 2005, 69(6):829-38.
8. Dereköy FS. Etiology of deafness in Afyon school for the deaf in Turkey. Int J Pediatr Otorhinolaryngol. 2000, 55(2):125-31.
9. Kittrell AP, Arjmand EM. The age of diagnosis of sensorineural hearing impairment in children. Int J Pediatr Otorhinolaryngol. 1997, 40(2-3):97-106.
10. Abdel-Hamid O, Khatib OMN, Aly A, Morad M, Kamel S. Prevalence and patterns of hearing impairment in Egypt: a national household survey. East Mediterr Health J. 2007, 13(5):1170-80.
11. Lee IWCC, Brasileiro HMS, Boldorini PR, Rapoport A, Novo NF. Perfil epidemiológico das deficiências auditivas no interior de São Paulo. Estudo de 234 casos. Rev Bras Cir Cabeça Pescoço. 2004, 33(2):89-92.
12. Veras RP, Mattos LC. Audiologia do envelhecimento: revisão da literatura e perspectivas atuais. Rev Bras Otorrinolaringol. 2007, 73(1):122-8.
13. Wilson DH, Walsh PG, Sanchez L, Davis AC, Taylor AW, Tucker G et al. The epidemiology of hearing impairment in an Australian adult population. Int J Epidemiol. 1999, 28(2):247-52.
14. Walch C, Anderhuber W, Köle W, Berghold A. Bilateral sensorineural hearing disorders in children: etiology of deafness and evaluation of hearing tests. Int J Pediatr Otorhinolaryngol. 2000, 53(1):31-8.
15. Streppel M, Richling F, Roth B, Walger M, von Wedel H, Eckel HE. Epidemiology and etiology of acquired hearing disorders in childhood in the Cologne area. Int J Pediatr Otorhinolaryngol. 1998, 44(3):235-43.
16. Política Nacional de Saúde da Pessoa Portadora de Deficiência [homepage na Internet]. Portaria n. 1.060, de 5 de junho de 2002. Diário Oficial, Brasília (2002 jun 10).[acesso em 2009 Out 29]. Disponível em: http://portal.saude.gov.br/portal/arquivos/pdf/manual2.pdf
17. Daniel E. Noise and Hearing Loss: A Review. J Sch Health. 2007, 77(5):225-31.
18. Fukuda Y. Otorrinolaringologia. 1ª ed. São Paulo: Manole, 2003.
19. Jafek BW, Murrow BW. Segredos em Otorrinolaringologia: respostas necessárias ao dia-a-dia em rounds, na clínica, em exames orais e escritos. 2ª ed. Porto Alegre: Artmed, 2006.
20. Hungria H. Otorrinolaringologia. 7ª ed. Rio de Janeiro: Guanabara Koogan,1995.
21. Butugan O, Santoro PP, Almeida ER, Silveira JAM, Grasel SS. Diagnóstico precoce da deficiência auditiva no primeiro ano de vida de crianças com alto risco através de audiometria de tronco cerebral. Pediatria (São Paulo). 2000, 22(2):115-22.
22. Brasil livre da rubéola: campanha nacional de vacinação para eliminação da rubéola, Brasil, 2008: relatório/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. - Brasília: Ministério da Saúde, 2009.
23. Secretaria de Vigilância em Saúde do Ministério de Saúde do Brasil [homepage na Internet]. Casos confirmados de Rubéola, Brasil, Grandes Regiões e Unidades Federadas 1997 a 2006 [acesso em 2009 Out 29]. Disponível em: http://portal.saude.gov.br/portal/arquivos/pdf/casos_rubeola.pdf.
24. Secretaria de Vigilância em Saúde do Ministério de Saúde do Brasil [homepage na Internet]. Casos confirmados de Rubéola, segundo mês de início de sintomas, Brasil, Grandes Regiões e Unidades Federadas 2007 [acesso em 2009 Out 29]. Disponível em: http://portal.saude.gov.br/portal/arquivos/pdf/casos_conf_rubeola_mes_sintomas2007.pdf.
1 Bachelor's Degree in Medicine from the Federal University of Santa Catarina.
2 Assistant Professor of the Federal University of Santa Catarina. Doctoral Degree in Otorhinolaryngology from the University of São Paulo.
3 Assistant Professor of the Federal University of Santa Catarina.
4 Otorhinolaryngologist Doctor. In course for Post-Graduation Degree from the Department of Otorhinolaryngology of the Federal University of São Paulo.
5 Epidemiologist - Professor of the Post-Graduation Program in Medical Sciences CCS/HU/UFSC. Doctoral Degree in Epidemiology from the University of London - United Kingdom.
Institution: Universidade Federal de Santa Catarina. Florianópolis / SC - Brazil. Mail address: Oscar Cardoso Dimatos - Avenida Jornalista Rubens de Arruda Ramos, 1010, Apto 601 - Centro - Florianópolis / SC - Brazil - Zip code: 88015-700 - Telephone: (+55 48) 3225-2002 / (+55 48) 9914-2002 - E-mail: zicodimatos@yahoo.com.br
Article received on October 21, 2010. Approved on November 13, 2010.
|
|
 |
|
|
