INTRODUCTIONPerforations of tympanic membrane (TM) are holes caused by structural alterations of tympanic tissue and are histologically characterized by epithelization of their borders(1).
The TM perforation treatment has focused to main lines of scientific research. The first one includes perforation cicatrisation through new protein matrix which provide support and guidance for the regenerating tissue (miringoplasty)(2,3). The second one aims at inducing the cell proliferation and migration through mitogens (growth modulator factor) or blood flow inductors (hemorrheologic agents).
The epithelium growth factor (EGF) is a cytokine which is able to modulate the proliferation and quimotaxis of epithelium, endothelium, fibroblast and keratinocyte cells(4-6). Specific receptors for the EGF have been detected in animal and human TM(7-9). Experimental studies and clinical essays have investigated the EGF effectiveness in promoting TM perforation cicatrisation. Some have observed statistically significant difference between the study group and the control group(7,10,11), while others haven't(12, 13).
The pentoxifilin (PX) is a metilxantina which is able to increase the blood flow and the oxygen intensity in tissues with deficient peripheral microcirculation(14). It has been used in the treatment of skin flaps and leg vein ulcers(15-17). However, the only experimental study about acute TM perforation has not been able to demonstrate benefits with the use of PX(18). The literature about this topic is controversial and lacks PX and EGF action objective quantification in TM perforation cicatrisation.
The initial treatment of TM traumatic perforations is the clinical observation; the surgical correction is only indicated in cases when spontaneous cicatrisation is not observed after a period of three months (19, 20). The main idea has been to experimentally test a simple and non-invasive method that may be an option on TM traumatic perforation which are not healed in their acute phase. Thus, we have chosen to study the effect of cicatrization inductor substances in a subacute phase of perforation, taking advantage of a possible residuous mitotic activity before the perforation has a surgical indication.
We have developed this experimental study to test the following hypothesis: the EGF and the PX increase the TM perforation cicatrisation percentage (CP) and the two drug use has synergic effect on the CP. In order to test such hypothesis this study objective has been to determine and quantify the EGF and PX effects, combined or isolated, on chinchilla TM subacute perforation cicatrisation.
METHODThis research project has been approved by Comissão de Ética para Análise de Projetos de Pesquisa (CAPPesq) (Ethics Comittee for Research Project Analysis) Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP) (São Paulo University Hospital), under research number 853/04. The experiment has been performed at the Laboratório de Investigação Médica (LIM - 32) (Medical Investigation Laboratory) of the Otorrinolaringology Department, according to the manual about care and use of laboratory animals(21). The research has been sponsored by reserach aid fee, approved by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), case number 02/08285-0.
42 male and female chinchillas have been initially selected, aging from 7 up to 20 months, weighing from 400g and 800 g, which have been obtained through specialized raisers (CHILLÁN - Criação de Chinchilas, Reprodutores e Matrizes / ACHILA - Associação Brasileira de Criadores de Chinchilas). The animals have been kept by their own raisers in individual cages with water and food as they wished. We have excluded animals which had infection on medium or external ear, pregnant females and the ones which had congenital malformation.
All animals have been submitted to procedures to create TM perforations in their both ears. The anesthetic induction has been done subcutaneous via, through the use of insulin syringe and needle for xilazine chloride injection at 2% (3 mg/kg) followed by quetamine chloride (17 mg/kg). The procedure has been performed totally under endoscope view, through the use of rigid endoscope with view angle of 30º and 2.7 mm of diameter.
The TM total area has been calculated through the use of a 0.5-mm-thick silicon model (Xomed Surgical Products, Jacksonville, FL, USA) made at the membrane shape in order to cover all its surface. The perforation has been standardized for all animals in size and shape, through the made of a second model, corresponding to half of the first model total area and to TM inferior quarters (Figure 1).
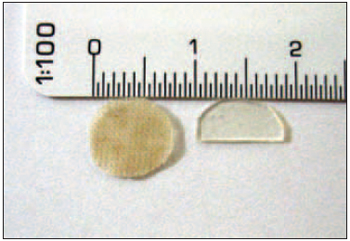
Figure 1. silicone model equivalent to the total area of tympanic membrane (left) and perforation model (right).
The perforation model has been placed on the TM inferior quarters. The miringotomy has been performed in the model limits through the use of needle extreme electrode thermal energy, at 90º on the distal extremity and 0.3 mm of diameter, connected to cautery knife (BP-100, EMAI, São Paulo, Brazil) adjusted in 15 w (Figure 2A). The remaining TM has been submitted to five perpendicular incisions to anulus. The tissue zones have had their borders medialized and cauterized to the inner layer (Figure 2B).
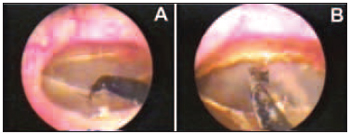
Figure 2. Miringothomy performed in the model limits using electrode (A) and cauterized and inverted perforation borders, meeting the inner layer (B).
The images have been obtained through the use of micro camera (Sony DXC-LS1) and endocoupler (22 mm) connected to the endoscope, imported from the video system to the computer. A graphic program (AutoCAD R14) provided the calculation of TM total area and of perforation area (Figure 3).
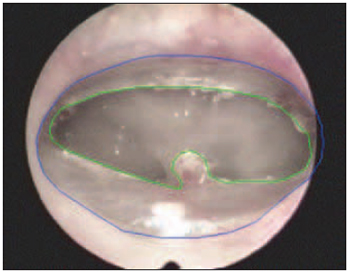
Figure 3. Calculation of tympanic membrane areas (blue) and perforation (green).
Profilaxy against infections has been done with enroploxacin at the 5 mg/kg/d dose oral via, at the first five days after the perforation and during the 10 days of perforation treatment.
After 30 days, the animals in which at least one year presented cicatrisation lower than 10% of percentage initial perforated area (PPA) have been included in the treatment. The infected ears have been excluded. The selected ears made four study groups: control group (received distillated water), EGF group (treated with EGF topic), PX group (received oral PX and distillated water) PX+EGF group (treated with topic EGF and oral PX).
One sterile sponge fragment of absorbing jelly (Gelfoam, Pharmacia Brasil Ltda, São Paulo, Brasil), manufactured according to TM model, of placed in order to take all TM remaining space. Topic solutions containing 25 µl of distillated water or 0.25 mg/ml of EGF (Biogen, Cambridge, MA, USA) have been applied on the sponge, using micropipette of 25 µl fixed volume (Socorex - Acura, Cral, Cotia, Brasil), every 72 hours for 10 days (total of three applications), after which the sponge has been removed (Figure 5). The PX has been given at 20 mg/kg/d dose, oral via, every 12 hours, for 10 days, coinciding with the period of topic solution use.
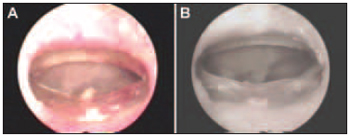
Figure 4. Tympanic Membrane Perforation on its day (A) and 30 days after the perforation procedure with cicatrisation lower than 10% (B).
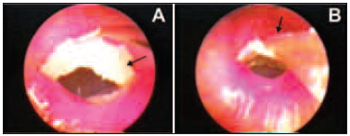
Figure 5. Jelly sponge (arrow) being placed over the remaining tympanic membrane (A) and micropippet extremity (arrow) used in the topic solution application (B).
The animals were put down in a CO
2 chamber for no more than 10 minutes(22). After the sacrifice, the animals have been placed into hospital waste.
The PPA has been calculated by dividing the perforated area by the TM total area and multiplying it by 100. The TM perforation PC in one month has been defined as the difference between the PPA in the beginning of the treatment and the one observed after 30 days.
The treatment final effect over the TM perforations has been classified into: absent cicatrization when the PC has been lower than 10%, partial cicatrization when the PC has been equal to or higher than 10% and total cicatrisation when perforation has not been observed 30 days after the beginning of the treatment.
The obtained data have been submitted to descriptive analysis. The PPA and the TM perforation PC have been calculated in each group. The categorical variables (treatment final effect on TM perforations) have been described through their frequency and compared among groups through the use of Fisher test. The continuous variables (PPA and PC) have been described through their averages and standard deviations and submitted to variation analysis as a factor ("one-way" ANOVA). The comparison of every two groups has been made through the Bonferroni "post-hoc" test. A statistical significance level of p
< 0.05 has been admitted. The statistical analysis has been performed through the use of statistical program (STATA 8.0 TM).
RESULTS The initial sample has been made of 42 animals (84 ears) which were submitted to standard procedures to create bilateral TM perforation. 30 days after the procedure, 38 ears (45.2%) have been excluded for partial or total perforation cicatrisation, for excessive cicatrisation retraction with rare residuals TM and infections, or for animal death during anesthetic induction (Figure 6). The final sample has been made of 46 ears (54.8%) whose perforation was healed less than 10% after 30 days and were included in the treatment record. During this phase, three animals were lost due to death during anesthetic induction and therefore six ears have been excluded from the data analysis. The 40 remaining ears (47.6%) have been distributed into the treatment group in the following way: 9 in the control group, 8 in the EGF group, 13 in the PX group and 10 in the EGF+PX group.
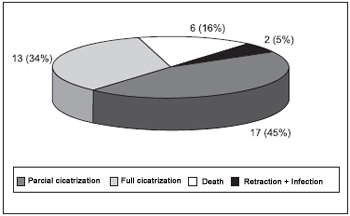
Figure 6. Causes of exclusion - 30 days after procedures.
As it may be observed on Figure 7, there has been total perforation cicatrisation in only one ear (10%) of EGF+PX group. In the same group the partial cicatrization happened in 7 ears (70%) and the absence of cicatrisation in 2 ears (20%). The 8 ears (100%) of the EGF group have presented partial cicatrisation, which also happened in five ears (55%) of the control group and in five ears (38.5%) of the PX group. The difference observed in the result distribution has been statistically significant through the Fisher test (p = 0.007).
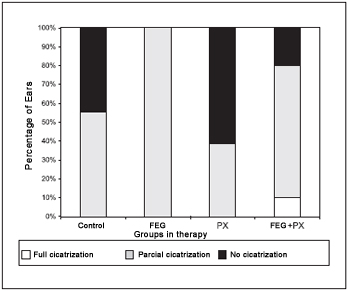
Figure 7. Percentage of cicatrization between groups on therapy.
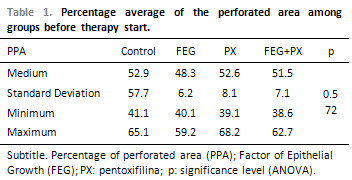
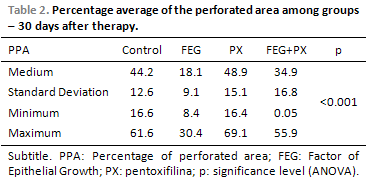
Before the beginning of the treatment, the groups did not present any difference among the PPA averages (ANOVA; p = 0.572; Table 1). At that time, the average PPA was of around 50%. Thirty days after the treatment, the groups presented statistically significant difference among the PPA averages (ANOVA; p < 0.001). The average PPA in the control and PX groups were higher than 44%, while the other groups presented much lower averages (Table 2). Picture 8shows PPA before and after 30 days of treatment. The comparison among every two groups have demonstrated that the average PPA after the treatment was significantly lower in the EGF group than in the control group (p = 0.003) or in the PX group (p < 0.001). There has been no difference among the PPA averages when control and PX groups (p = 1.00); control and EGF + PX groups (p = 0.964); EGF and EGF + PX groups (p = 0.097); and PX and EGF + PX groups (p = 0.142) were compared.
Concerning the PC averages in the TM perforations, we observe statistically significant difference among the treatment groups (ANOVA; p < 0.001; Table 3). The average PC has remained lower than 9% in the control and PX groups and higher than 16% in the other groups. The comparisons in the study groups, every two groups, have demonstrated that the PC average in the EGF group has been significantly higher in the PX and control groups (p = 0.003 e < 0.001 respectively). The average PC in the EGF+PX group has presented tendency to difference, but not statistically significant, when compared to EGF group (p = 0,101) and PX group (p = 0,069). There has not been statistically significant difference in the average PC when the control and PX group were compared (p = 1.00) and the EGF+PX and control group were compared (p = 0,874), Figure 9.
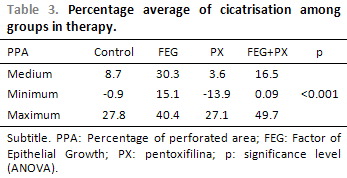
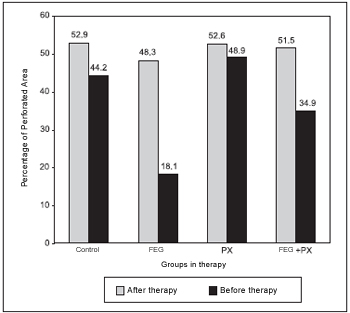
Figure 8. Percentage of perforated area among groups before and after therapy.
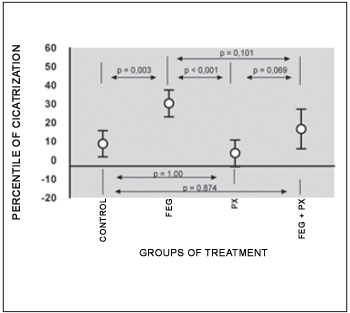
Figure 9. Average cicatrization percentage of TM perforation (average standard deviation).
Little more than half of our initial sample presented TM perforations with cicatrization inferior than 10%, four weeks after the perforation procedure. Amoils et al.(23), using a similar model, have had higher non-healed TM perforation proportion (84%), though it had different inclusion criteria. In our sample the ears have been rigorously selected for treatment and in any situation the perforation procedures with spontaneous cicatrisation has been repeated, a technique that has been adopted by such authors. On the other hand, Dvorak et al.(12), using an animal model which was identical to the one described by Amoils et al.(23), have obtained 31% of non-healed TM perforation.
In a retrospective study, Griffin(19) observes that 70% of MT traumatic perforations heal spontaneously in 30 days and such cicatrization rate drops to 18% in the following months. The perforations made in chinchillas TM in this study may be considered subacute once after 30 days of observation we were careful to exclude the ears with equal or higher than 10% PC. Such criteria has been adopted in order to cover any margin of error in the measuring and to guarantee that the events observed after this period could be attributed only to the discussed intervention. Besides, in a previously issued study, we have performed a histologic and imunohistochemical analysis of 16 TM, 30 days after the perforation procedure(24). The observed results have showed absence of myofibroblasts which expressed the
a-actine pf plain muscle, a cell which is indispensable in the contraction of any healing tissue, which suggests chronification of the process with reacutization eruptions, which reinforces the subacute nature of induced perforation by the animal model which has been used by us(24).
The treatment final effect over the TM perforations has been different among the groups. The partial and/or total cicatrisation has been twice more frequent in EGF and EGF+PX groups than in the other ones, while the absence of cicatrisation has been more frequent in the control and PX groups. Our findings can not be directly compared to the ones by other authors(10-12), once they deal will perforations which are considered chronic, used two study groups (with or without EGF) and consider only the total TM cicatrisation the study closure. However, we agree with Amoils et al.(10) and with Lee et al.(11) that cicatrisation is more probable when EGF is used.
The average PPA and its standard deviation before the treatment have been similar among the groups, assuring that the TM perforations had approximately the initial size. The statistically significant difference in the PPA average, 30 days after the treatment, among the groups, shows that the used drugs have influenced in several different ways in the TM perforation cicatrisation.
The EGF, in the used posology, promoted a TM perforation PC 1.8 times higher than in the EGF+PX group, 3.5 times higher in the control group and 8.4 times higher in the PX group. The PC in each group represents a period of 30 days, considering that the initial PPA was of approximately 50% and supposing that such PC remains continuous, the cicatrisation would be complete in 1.5 months after the beginning of the treatment in the EGF group, in 3 months in the EGF+PX group, in 5.5 months without any treatment and in 12,5 months in the PX group.
The experimental studies which have tested the EGF in chronic TM perforations have showed conflicting results. Amoils et al.(10) and Lee et al.(11) have observed that the TM perforation percentage with complete cicatrisation has been 3 to 4 times higher in the EGF group than in the control group, while Dvorak et al.(12) have not been able to reproduce such results. Part of such discrepancy may be due to the mechanical trauma and to the induced bleeding in the TM during the repeated jelly sponge exchanges (more than six times per ear), which may have promoted the spontaneous cicatrisation in the control group.
Despite the EGF has not been liberated for human use, Ramsay et al.(13) have performed an open clinical essay in which patients with chronic medium otitis have been treated with EGF. However, they have observed similar medium cicatrisation of TM perforation among the study groups and placebo. It is possible that part of such result is due to the small doses used and to the short period of exposure to drug.
The idea of combining EGF and PX has been based on the fact that every tissue needs enough oxygenation and vascularization so that mitosis and cell replication occur, and that anoxia and endoxines inhibit such events(25). Our hypothesis was that the use of a blood flow inductor, such as the PX, could potentialize the EGF mitogenic action in the TM cicatrisation. However, we have not observed any benefit with the use of PX in TM perforation in chinchillas. On the contrary, we have observed lower TM PC in the groups which have received PX. Such PC has been around twice lower in the EGF+PX group when compared to the EGF group and 4.5 times lower in the PX group when compared the the EGF+PX group, with tendency to a statistically significant difference by the "post-hoc" test. That suggests that PX probably interferes negatively in the EGF action. Some publications have demonstrate that the PX cell effects, specially the phosphodiesterase, are not restrict to the eritrocits (26,27). The increase in the intra-cellular concentrations of cyclic adenosine monophosphate occurs in other cells such as fibroblasts and keratinocytes which have been treated with PX, which leads to cell proliferation inhibition, probably by lower modulation of cytokines action and by nuclear transcription factors(26,27).
Jull et al. in a systematic review about the PX effects in chronic skin ulcers have concluded that such drug is 41% more effective than the placebo in the cicatrisation of such lesions. Although our studies may not be compared to such authors', our initial expectation with PX benefit was much higher. It is possible that the good results observed by them(17) may be partly attributed to the drug anti-inflammatory and anti-fibrogenic effects, a fact which has been discovered recently(28,29). According to such authors, the PX is able to reduce the pro-inflammatory cytocine production and the fibrogenesis inductor factor production, limiting the cell proliferation(28, 29).
Our results are not comparable to Lim et al.'s results(18), once they have used PX in an experimental model of TM acute perforation in guinea pigs, with great potential of spontaneous cicatrisation, which partly harms the drug isolated action evaluation. However, as well as such authors, we have not observed any benefit with the use of PX in TM perforation.
CONCLUSION The EGF assisted the cicatrisation process of TM subacute perforation in chinchillas, which has not been observed with the PX. The EGF and the PX combination have not potentialized its effect.
REFERENCES 1. Bento RF, Miniti A, Marone SAM. Tratado de otologia. 1a ed. São Paulo: EDUSP; 1998.
2. Laidlaw DW, Costantino PD, Govindaraj S, Hiltzik DH, Catalano PJ. Tympanic membrane repair with a dermal allograft. Laryngoscope 2001;111(4 Pt 1):702-7.
3. Oliveira JAA, Hyppolito MA, Netto JC, Mrué F. Myringoplasty using a new biomaterial allograft. Rev Bras Otorinolaringol. 2003;69(5):649-655.
4. Carpenter G, Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol 1976;88(2):227-37.
5. Aharonov A, Pruss RM, Herschman HR. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem 1978;253(11):3970-7.
6. Bennett NT, Schultz GS. Growth factors and wound healing: Part II. Role in normal and chronic wound healing. Am J Surg 1993;166(1):74-81.
7. ODaniel TG, Petitjean M, Jones SC, Zogg J, Martinez SA, Nolph MB, et al. Epidermal growth factor binding and action on tympanic membranes. Ann Otol Rhinol Laryngol 1990;99(1):80-4.
8. Mondain M, Ryan A. Epidermal growth factor and basic fibroblast growth factor are induced in guinea-pig tympanic membrane following traumatic perforation. Acta Otolaryngol 1995;115(1):50-4.
9. Somers T, Goovaerts G, Schelfhout L, Peeters S, Govaerts PJ, Offeciers E. Growth factors in tympanic membrane perforations. Am J Otol 1998;19(4):428-34.
10. Amoils CP, Jackler RK, Lustig LR. Repair of chronic tympanic membrane perforations using epidermal growth factor. Otolaryngol Head Neck Surg 1992;107(5):669-83.
11. Lee AJ, Jackler RK, Kato BM, Scott NM. Repair of chronic tympanic membrane perforations using epidermal growth factor: progress toward clinical application. Am J Otol 1994;15(1):10-8.
12. Dvorak DW, Abbas G, Ali T, Stevenson S, Welling DB. Repair of chronic tympanic membrane perforations with long-term epidermal growth factor. Laryngoscope 1995;105(12 Pt 1):1300-4.
13. Ramsay HA, Heikkonen EJ, Laurila PK. Effect of epidermal growth factor on tympanic membranes with chronic perforations: a clinical trial. Otolaryngol Head Neck Surg 1995;113(4):375-9.
14. Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987;34(1):50-97.
15. Nemiroff PM. Synergistic effects of pentoxifylline and hyperbaric oxygen on skin flaps. Arch Otolaryngol Head Neck Surg 1988;114(9):977-81.
16. Armstrong M, Jr., Kunar DR, Cummings CW. Effect of pentoxifylline [corrected] on myocutaneous flap viability in pigs. Otolaryngol Head Neck Surg 1993;109(4):668-75.
17. Jull AB, Waters J, Arroll B. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2002(1):CD001733.
18. Lim AA, Washington AP, Greinwald JH, Lassen LF, Holtel MR. Effect of pentoxifylline on the healing of guinea pig tympanic membrane. Ann Otol Rhinol Laryngol 2000;109(3):262-6.
19. Griffin WL, Jr. A retrospective study of traumatic tympanic membrane perforations in a clinical practice. Laryngoscope 1979;89(2 Pt 1):261-82.
20. Bento RF, Lessa MM, Chung D, Wiikmann C, Miniti A. Condutas práticas em otologia. 1a ed. São Paulo: Fundação Otorrinolaringologia; 2002.
21. NationalResearchCouncil. Manual sobre cuidados e usos de animais de laboratório. 1a ed. Goiânia: National Academy Press; 2003.
22. Souza NL. Eutanásia. In: Luca RR, Alexandre SR, Marques T, Souza NL, Merusse JLB, Neves SP, editors. Manual para técnicos em bioterismo. 2a ed. São Paulo: FINEP-COBEA; 1996. p. 157-177.
23. Amoils CP, Jackler RK, Milczuk H, Kelly KE, Cao K. An animal model of chronic tympanic membrane perforation. Otolaryngol Head Neck Surg 1992;106(1):47-55.
24. Ramalho JR, Bento RF. Healing of subacute tympanic membrane perforations in chinchillas treated with epidermal growth factor and pentoxifylline. Otol Neurotol 2006;27(5):720-7.
25. Bankey P, Fiegel V, Singh R, Knighton D, Cerra F. Hypoxia and endotoxin induce macrophage-mediated suppression of fibroblast proliferation. J Trauma 1989;29(7):972-9; discussion 979-80.
26. Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol 1989;92(4):605-10.
27. Gilhar A, Grossman N, Kahanovicz S, Reuveni H, Cohen S, Eitan A. Antiproliferative effect of pentoxifylline on psoriatic and normal epidermis. In vitro and in vivo studies. Acta Derm Venereol 1996;76(6):437-41.
28. Stosic-Grujicic S, Maksimovic D, Badovinac V, Samardzic T, Trajkovic V, Lukic M, et al. Antidiabetogenic effect of pentoxifylline is associated with systemic and target tissue modulation of cytokines and nitric oxide production. J Autoimmun 2001;16(1):47-58.
29. Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, Riecken EO, et al. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut 2002;50(2):241-7.
Doctor's Degree (Doctor On Sciences By The Oftalmology And Otorrinolaringology Department Of São Paulo University)
Jeanne Oiticica Ramalho
Adress: Av Damasceno Vieira, 1202, apto 41 - CEP: 04363-040 - São Paulo/SP - Brasil - Tel/Fax: +55 11 56716810 - E-mail: jeanneramalho@uol.com.br.
Fundação De Amparo À Pesquisa Do Estado De São Paulo (Fapesp) (Research Aid Foundation Of São Paulo State)
This article was submitted at Publication Management System of R@IO on Sep. 10th, 2006 and approved on January 26th, 2007, 20:30:48