INTRODUCTION Nasal Polyposis affects from 1% to 4% of population(1,2) with more frequency in men(3,4) especially over 50(3) and rarely affecting children and young people. When it is associated with asthma, they affect it affects more frequently females(4). Its origin is still uncertain. It is probably multifactorial, because its causes are by bacteria or structure alteration infections such as septum deviation and anatomical variations of medium meatus which causes inflammation and then Nasal Polyposis(1). It is distinguished by an inflammatory chronic process of nasal respiratory mucosa and of paranasal sinus(1).
Histologically, it presents itself with large quantity of inflammatory cells such as lymphocytes, mastocytes, basophiles with predominance of eosinophiles, and non-inflammatory cells such as fibroblasts and epithelial cells. Besides, there is presence of inflammatory mediator as cytokines and growth factors which are active in the region(1,5).
Nasal Polyposis has a high rate of morbidity besides determining an expressive impact on patient's quality of life, as mentioned in other studies(2,6,7,8). Its symptoms is related to alteration on sinusal function leading to anosmia, nasal obstruction, anterior and posterior rhinorrhea, sneezing and local itching(8,9), yet it is also related to sleeping disorders, headaches and anger. The clinical triad, Nasal Polyposis, Asthma and Aspirin Sensitiveness is a condition of morbidity, by representing the most aggressive way of the disease(3). Though, it is not completely found in all patients who suffer from Nasal Polyposis.
Nasal Polyposis should be considered as a multifactorial disease(2), as it is often associated to atopic individuals, patients with asthma, chronic rhinossinusopathy, mucocilliary motility disorder, non-steroidal anti-inflammatory sensitiveness and cystic fibrosis(3,10).
The current study evaluates patients' symptoms who suffer from Nasal Polyposis and its related co-morbidity, such as asthma and aspirin sensitiveness, and also to review some of aspects of the disease.
MATERIALS AND METHODS A prospective study was done with 24 patients suffering from Nasal Polyposis when followed-up at Serviço de Otorrinolaringologia do Hospital das Clínicas do Paraná. All patients were submitted to a questionnaire and supplementary evaluation by Computed Tomography Scan. The study was approved by Comitê de Ética em Pesquisa em Seres Humanos (Ethics Commitee on Human Being Research) on May 11th, 2007 under #1343.008/2007-01.
Age, gender, profession, work pollutant, smoking habits and aspirin sensitiveness were evaluated, according to clinical history reported by patients. Regarding nasal symptoms, the presence or absence of them were equally evaluated, such as rhinorrhea, nasal obstruction, nasal itching and sneezing and conjunctivitis, besides hypo or anosmia complaints.
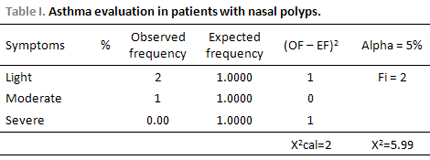
Patients with asthma were classified according to disease intensity. The ones with mild asthma reported symptoms during less than twice a week which were of short duration, with absence of night symptoms or present less than twice a month, being asymptomatic between the exacerbations. The ones with moderate asthma reported symptoms more than twice a week and more than twice a month during the night. Patients with severe asthma reported often symptoms during the day and night, previous crisis occurrence threatening their lives, limited physical activity and one hospitalization in the last 12 months at least.
Tomography evaluation of patients with Nasal Polyposis was performed according to Kennedy et al(3): Grade 1: bilateral ethmoid disease. Grade 2: bilateral ethmoid disease affecting one paranasal sinus. Grade 3: bilateral ethmoid disease affecting two or more paranasal sinus. Grade 4: diffuse rhinosinusal polyposis.
The Qui-Squared Test was applied for the statistical analysis. The value p<0.05 was established as a rejection level of hypothesis of nullity.
RESULTSFrom the 24 evaluated patients, 41.66% was male. Age raged from 50 to 59 year, corresponding to 37.5% of the cases, followed by patients aging from 40 to 49, which represented 29.16% (Picture 1). Only 12.5% (3/24) of patients presented asthma, 2 of them suffered from light asthma and only 1 suffered from moderate asthma. No patients suffered from severe asthma (Table 1). Regarding aspirin sensitiveness, 8.33% (2/24) of patients with Nasal Polyposis reported such sensitiveness, and suffered from asthma. Regarding smoking habits, 33.33% (8/24) smoked 15 packs/year in average.
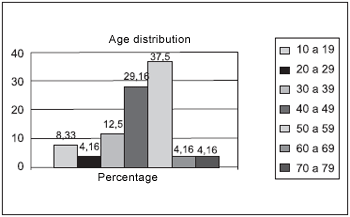
Picture 1. Age distribution of evaluated patients with nasal polyps.
The most frequent clinical manifestations were anterior and posterior rhinorrhea and anosmia. Both were present in 90% of the patients who were submitted to the questionnaire. 80% of patients reported nasal obstruction; 60% reported sneezing; 50% reported itching and 30% of them reported conjunctivitis (Picture 2 and Table 2).
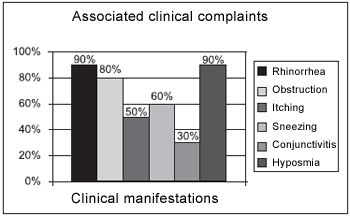
Picture 2. Symptoms in patients with nasal polyps.
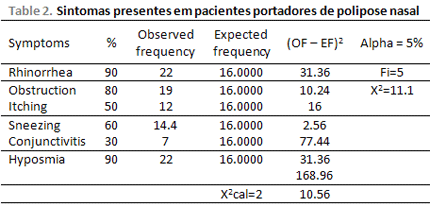
Regarding tomography evaluation of the nasossinusal disease, 45.83% of patients was classified as Grade 3 (bilateral ethmoid disease affecting two or more paranasal sinus of each side), 29.16% was classified as Grade 2, and 12.5% was classified as Grade 1 and 4 respectively (Table 3). The correlation between severity of the disease by Computed Tomography Scan and clinical symptoms was statistically significant from Qui-Squared Test, with p-value = 0.04. Asthma severity was related to tomography class, as all patients were classified in grade 3 or 4 (p<0.05).
DISCUSSION The features of the evaluated patients were similar to the ones in the literature. Patients' age ranged from 50 to 59 years (37.5%). Bonfils et al. reported an average of 49 years old and Matsuyama reported 50 years old. Association between Nasal Polyposis and asthma is commonly reported in the literature (around 7% to 20%(2)). Asthma affects 45% of patients with Nasal Polyposis (11.12%). In the current study, the figure is 12.5%, what can be explained by the evaluated samples. On the other hand, Nasal Polyposis affects from 5.2% to 13%(11,12) of patients with asthma. Pathology in patients with asthma is usually more severe and refractory to treatment when compared to patients without asthma.
Klossek et al(13), during a pre-surgical evaluation on patients holding Nasal Polyposis, observed a predominance of 25.5% of asthma and of 29,7% of ASA, a clinical syndrome which is Aspirin Sensitive Asthma. The relation between ASA and Nasal Polyposis is described(14), and in most of the patients with ASA, the anterior rhinoscopy and the tomography study reveal the presence of nasal polypus(10). The high predominance of Nasal Polyposis in patients with ASA would be related to alterations on the HLA genes(2).
Klossek et al reported bilateral nasal obstruction and anosmia for 6 months in 100% of cases. In the current study, anterior and posterior rhinorrhea and anosmia affected 90% of patients.
In relation to the 8.33% of the patients who hold aspirin intolerance, we have observed that such values agree to the results obtained by Bateman et al, in which 5% to 10% of the patients who hold Nasal Polyposis have intolerance to Acetylsalicylic Acid.
Nasal Polypi are evaginations of the nasal mucosa that are originated from the etmoidal sinus, medium turbinate maxillary sinus(10). Due to the frequent localization of polypi on the osteomeatal complex, the drainage and the aeration become deficient, thus frequently leading to acute clinical pictures of infection with the presence of purulent rhinorrea, fever and night cough, among other symptoms(3).
Histologically, the nasal polypi are made of infiltrate of inflammatory cells with lymphocytes, mastocytes, basophiles - and the eosinophiles represent more than 60 % of the cells - in addition to non-inflammatory cells such as fibroblasts and epithelial cells(2). There is production of great quantity of cytokines and growth factors, which contribute to the chronic eosinophilic inflammation regulating the migration, survival and activation of eosinophiles (15). The cytokines growth factors such as interleukins 3, 4 and 5, and Interferon-gama and TGF-
b1 (
b1 factors of transgenic growth) are synthesised by lymphocytes T, fibroblasts, epithelial cells and eosinophiles. Such factors are expressed in higher or lower quantity according to individual atopy(17,18) and should be liable to different phases in the polyp formation process(1), thus characterizing different phases of Nasal Polyposes. Such interlukinas promote deposit of collagen of kind I, II and V, thus contributing for the thickening of the local bacal membrane, estromal fibrosis and epithelial hyperplasia thus bringing underepithelial remodeling(1, 17, 18).
Different histological Standards of Nasal Polyposis may be found(2). The nasal polypi may be made of normal or abnormal respiratory epithelium, made of respiratory mucosa and transition epithelium areas which contain scaly cells, so undermucous glands may be present in equal or lower quantity when compared to the nasal mucosa. Metaplasic histological standards may also be found(1, 17, 18).
Such inflammatory standard described in the Nasal Polyposis is similar to the one demonstrated in the asthma physiopathology, which proves the co-relation between both pathologies(4). The higher the remodeling due to fibrosis, the higher the severity of the disease(13, 17). Such affirmation has been proved in this study by the co-relation of tomography severity of the disease and the presence of asthma, once all patients who hold asthma were in degree 3 or 4 of the tomography classification by Kennedy et al.
The Nasal Polyposis is also related to the metabolism of prostanoids (2,10). It has already been demonstrated that there is a delay in the activation of Ciclooxygenasis-2 (COX-2) in the nasal polypi when compared to the nasal mucosa. Such fact leads to a deficiency in the production of Prostagladines, which are involved with the modulation in the nasal mucosa. The deficiency in its production would be a reason for the polypi formation(2).
It has been demonstrated that in patients with ASA the COX-2 levels produced by nasal polypi are even smaller than in nasal polypi of patients who hold aspirin tolerance, thus demonstrating that the lack of prostaglandins in such patients makes them more sensitive to non-steroidal anti-inflammatory drugs effects(2).
The complementary evaluation through CT scan is practically obligatory(3) for all patients with Nasal Polyposis, which provides the inference based on the classification used the degree of morbidity and severity of the disease, in addition to its extent. Kerem et AL report that among patients holding Nasal Polyposis in his pre-operatory study of endoscopy surgery, 94.2% remained in the levels 3 or 4 of tomography sinus disease severity(15). In the present study, 58.7% of the patients belonged to the groups 3 or 4 according to the tomography evaluation.
CONCLUSION Although Nasal Polyposis is a frequent disease, its etiology is still unknown. The presence of eosinophilia, mastocyte degranulation and high levels of IgE in its histopathological evaluation suggest an allergic component in its etiology. The association between polyposis and asthama and aspirin intolerance is already recognized. The present study suggest the co-relation between the polyposis severity and its association with asthma, once all patients holding asthma were found in levels 3 or 4 of the tomography classification by Kennedy et al. However, due to the small sample size, all studies which possibly confirm such findings are necessary.
REFERENCES 1. Souza BB, Serra MF, Dorgam JV, Sarreta SM, Melo, VR, Anselmo-Lima W. Polipose Nasossinusal: Doença Inflamatória Crônica Evolutiva? Revista Brasileira de Otorrinolaringologia 2003; 69(3):318-325.
2. Puwankar R. Nasal Polyposis: An Update. Curr Opin Allergy Immunol 2003; 3:1-6
3. Matsuyama C. Polipose nasossinusal: Uma Revisão Bibliográfica Revista Brasileira de Otorrinolaringologia, 2000; 7(3):82-84.
4. Collins M, Pang Y-T, Loughran S, Wilson J.A. Environmental Risk Factors and Gender in Nasal Polyposis. Clin Otolaryngol 2002; 27:314-317.
5. Bateman ND, Fahy C, Woolford J. Nasal Polyps Still More Questions Than Answers. T J Laring. Otol, 2003; 117(1):1-6.
6. Alobid I, Benitez P, Bernal-Sprekelsen M, Morello A, Picado C, Mullol J, Wang M, Goodwin J. Impact of Nasal Polyposis and Its Medical or Surgical Treatment on Quality of Life. Otolaryngology - Head and Neck Surgery, August 2004; R137, P196.
7. Alobid I, Benitez-Silva P, Bernal-Sprekelsen M, Morello A, Picado C, Mullol J.Severe Nasal Polyposis and its Impact on Quality of Life. Otolaryngology - Head and Neck Surgery, August 2005; P256:P178.
8. Radenne F, Lamblin C, Vandezande L-M, Tillie-Leblond I, Darras J, Tonnel A-B, Wallaert B. Quality of Life in Nasal Polyposis. J Allergy Clin Immunol 1999; 103:79-84.
9. Bonfils P, Avan P, Nores JM. Corticosteroid Treatment in Nasal Polyposis with Three-Year Follow-Up Period. Laryngoscope, 2003; 113(4):683-687.
10. Picado C. Nasal Polyposis. Clinical and Experimental Allergy Reviews 2001; 1(2): 163-165 11. Slavin RG. Asthma and Sinusitis. J Allergy Clin Immunol 1992; 90: 534-7.
12. Senior BA, Kennedy DW. Management of sinusitis in the Asthmatic Patient. Ann Allergy Asthma Immunol 1996;77:6-19.
13. Klossek JM, Peloquin L, Friedman WH, Ferrier JC, Fontanel JP. Diffuse Nasal Polyposis: Postoperative Long-Term Results After Endoscopic Sinus Surgery and Frontal Irrigation. Otolaryngology-Head Neck Surgery 1997; 117:355-61.
14. Bochenska-Marciniak M, Grzelewska-Rzymowska I, Kuna P. Incidence of Nasal Polyposis in patients with Aspirin- Induced Asthma (ASA). J Allergy Clin Immunol 2000; 105;1(2):319.
15. Kerem GV, Barzilai E G. Functional Endoscopic Sinus Surgery in the Treatment of Massive Polyposis in Asthmatic Patients. T J Laringol and Otolog.2002; 116(3):185-189.
16. Kennedy DW. Prognostic Factors, Outcomes and Staging in Ethmoid Sinus Surgery. Laryngoscope; 1992; 102(suppl57): S1-18.
17. Mollet S, Hamid Q, Hamilos D. IL-11 and IL-17 Expression in Nasal Polyps: Relationship to Collagen Deposition and Supression by Intranasal Fluticasone Propionate. Laringoscope, 2003;113(10)1803-1812.
18. Vento SI, Ertama LO, Hytonen ML, Wolff CH, Mainberg CH. Nasal Polyposis: Clinical Course during 20 years. Ann Allergy, Asthma Immunol 2000; 85:209-14.
19. Guilemany J M, Benitez P, Alobid I, Bernal-Sprekelsen M, Picado C, Mullol J. Prevalence of Atopy to Commom Aeroallergens and Moulds in Patientes with Nasal Polyposis. Otolaryngology - Head and Neck Surgery, August 2004; P192: R126.
1. PhD Professor in Anatomy Department and Physician in ENT Service at Universidade Federal do Paraná - Federal University.
2. Resident Student Doctor in the 2nd year of ENT Service at Hospital de Clínicas da Universidade Federal do Paraná - Clinical Hospital of Federal University.
3. Academic Student in the 6th year of Medical School at Universidade Federal do Paraná.
4. PhD Titular Professor of ENT Discipline at UFPR. Head of ENT Department at Hospital de Clínicas da Universidade Federal do Paraná.
Serviço de Otorrinolaringologia do Hospital de Clínicas da Universidade Federal do Paraná - ENT Service of Clinical Hospital at Federal University.
Andrea Thomaz Soccol
Address: Rua General Carneiro, 181, 5º andar anexo B - Curitiba/PA - CEP: 80060.900 - Phone (41) 3360-1800 - E-mail: andreasoccol@yahoo.com.br
This article was submitted to SGP (Sistema de Gestão de Publicações - Publication Management system) of R@IO on October 20th, 2007 and approved on July 18th 2007 at 22:07:18.