INTRODUCTION It is important for this work, a moreover brief description of the recurrent laryngeal nerve or inferior laryngeal nerve. This nerve emerge from the vagus nerve with a path within the carotid sheath, between the carotid artery and internal jugular vein. Near the aortic arch in the mediastinum area, the left-vagus nerve branches off to form the left recurrent laryngeal nerve that surrounds the inferior aortic arch, and reaches the groove between the trachea and the esophagus and then rises to enter the larynx, between the cricoid cartilage and the thyroid cartilage. This nerve enters the side-back cricoarytenoid muscle. On the right side, near the subclavian artery, the vagus nerve gives rise to the right recurrent laryngeal nerve, in which, in the same way happens on the left recurrent laryngeal nerve, runs along the tracheoesophageal groove, reaches the larynx and enters through the side and back, the posterior cricoarytenoid muscle .
The recurrent laryngeal nerves innervate all the intrinsic muscles of the larynx, except for the cricothyroid muscle. This receives an external branch of the superior laryngeal nerve. It is important to mention that, the muscles with fixation in the larynx can be classified as extrinsic or intrinsic. The extrinsic muscles have an attachment point outside and another insertion in the laryngeal cartilages. This group of muscles is part of the suspensory system of the larynx itself. Intrinsic muscles have all their own inserts located in structures of the larynx. These muscles are responsible for the articulatory movements between the cartilages. They include, on the outer wall of the larynx, the cricothyroid muscle, and in the internal spaces, the interarytenoidal muscles, thyroarytenoid, vocal, posterior and lateral cricoarytenoid. To form the body of each vocal fold, according to HIRANO (1), it is possible to find the vocal muscle.
The vocal muscle was considered, before the Nomina Anatomica of 1998, as the inner portion of the thyroarytenoid muscle, and nowadays, it is classified as an independent muscle.
The laryngeal nerves of the domestic pig (Sus scrofa domestica), have, in general appearance, the same source, branching off and distribution presented by human laryngeal innervation (ALAJMO and SALIMBENI (2)). The superior laryngeal nerve (SLN) emerges from the vagus nerve in the nodose ganglion, and divide itself into two branches: the external laryngeal nerve (ELN) and the internal laryngeal nerve (ILN). The internal branch is a continuity of the superior laryngeal nerve, in which, in the side-back area of the thyroid membrane penetrates the larynx to ensure supraglottic sensitivity. The external branch is thinner and passes though the outside to reach the cricothyroid muscle (CT). The recurrent laryngeal nerve (RLN) emerges from the vagus nerve, working as an inferior laryngeal nerve through the side-back area of the cricothyroid membrane, between the cricoid arch and the thyroid cartilage lamina. According to STAVROULAKI & BIRCHALL (3), the recurrent laryngeal nerve is responsible for the motor function of all intrinsic muscles of the larynx, except for the cricothyroid muscle. It is also important to mention that the recurrent laryngeal nerve provides the pore flow of the vocal fold mucosa, with sensitive fibers.
Just like humanes, the left RLN, right after branching off from the vagus nerve, it passes under the aortic arch (Figure 1E) and reaches the tracheo-esophageal junction area, where it rises. The right RLN branches off at the highest point and evolves under the right subclavian artery (Figure 1F), also rising in the tracheo-esophageal junction (Figure 1D).
KURITA et al (4) studied the vocal folds of ten animal species using the human vocal folds as a standard reference to morphological comparisons. The author demonstrates that pigs have the vocal folds with dimensions similar to human beings, and in comparison to other species, the mechanical properties of the vocal folds in swines are those that can be better evaluated and compared to the vocal folds of human beings. Although the author does not emphasize that this fact allows the inference that pigs can be definitive and experimental models suitable for speech. The length of the membranous portion of the glottis, in adult male pig measured eighteen millimeters compared to fifteen millimeters found, on average, in the pore humane (male) membrane glottis. Joining the epithelium to the own lamina of the laryngeal mucosa, the pig displayed a thickness of 0.9 mm and man present 1.1 mm. according HIRANO (1), In the stratigraphy of the own lamina of the adult man, it was found three layers: surface layer, also known as Reinke's space, composed of flacid connective tissue; intermediate layer, with predominance of elastic fibers and the deep layer, with predominance of collagen fibers. In the own lamina of adult pigs (KURITA (4)), it was found in the laryngeal mucosa two little distinguishable layers, labeled as the surface layer, where prevails elastic fibers, and in the deep layer, where prevails collagen fibers.
According to GORTI et al (5), the porcine larynx is a cylindrical structure consisting of cartilaginous walls, perichondrium, skeletal muscle, conjunctive tissue and mucosa. It is possible to observe that spaces in the endolarynx can be divided, such as the humane larynx: glottic area, including vocal folds and its rhyme; supraglottic area, including ventricle, vestibular folds and vestibule; infraglottic area, inferior and posterior to the glottis, until reach the base of the cricoid cartilage.
A laryngeal skeleton in swines is very similar to the humane laryngeal skeleton. There are three single cartilages which are the epiglottis, the thyroid and the cricoid, and other two pairs cartilages, known as arytenoid and corniculate.
It should be emphasized that the intrinsic muscles are similar to humane laryngeal muscles. The only difference is in the interarytenoidal muscle that is bulkier in swines (GORTI et al (5)). As it happen to horses, dogs, monkeys and rabbits, the pig is among the animals used in experiments, which have laryngeal ventricle, feature that defines the presence of vestibular fold (KURITA et al (4)).
According to GORTI et al (5) the framework associated with KURITA 's et al's (4) information modified by the ones who performed this study, it presents differences and similarities between the porcine larynx and the humane larynx.
STAVROULAKI & BIRCHALL (3) state that detailed information on a pig's anatomy had become important. Several factors interact to increase the importance of this knowledge. In laryngology, it is possible to classify these factors under different angles, for example, in the research on transplantation of the larynx, in the research in speech, and in the research of laryngeal diseases.
Pigs are easily available, because they have ability to "speak", a submissive behavior, robust morphology and are easy to make hygienic. The disadvantage of using pigs as models appears in researches in which they must be kept in the lab for a few months. In these cases, the pigs can reach a body mass difficult to handle and it becomes difficult to give medicines or food.
In researches of the speech, the larynges of dogs have been used as an animal model for over two centuries. However, from a structural perspective, the porcine larynx has a phonation model that is more advantageous than the canine larynx (JIANG et al (6)).
STAVROULAKI and BIRCHALL (3) concluded that the general innervation of the pig resembles the innervation of human in origin, branching and general distribution. in a comparative study of laryngeal innervation in humans, pigs, dogs, cats and rats.
In transmission electron microscopy, by using the fixation technique with glutaraldehyde and forming contrast with lead citrate and osmium tetroxide, the classical sarcomeric organization of skeletal muscles is clearly visible and defined. Each clear or isotropic band (band I) is crossed by a line or disc called Z. The dark band or anisotropy (band A) presents the H band, which has the M line (Figure 2A). Among the fields of miofibrils, there are accumulations of glycogen granules (Figure 2D).
In mammals, mitochondrion are located mainly at the band I area, wrapping itself in the periphery of the miofibril (Figure 2B). They appear in large numbers and intermingled with miofilaments. This is visible in longitudinal sections and especially in transverse sections, in which is clearly possible to notice, the mitochondrion distribution in close proximity to the miofilaments. Mitochondrion of normal skeletal muscle present clearly visible ridges.
The cisterns of sarcoplasmic reticulum - SR and the sarcolemma tubule form triads clearly visible and distinguishable located at the junction of the bands A with the bands I (Figure 2C). Glycogen comes in dark granules and in sufficient quantity, especially in close relation to the miofilaments (Figure 2D). The always multiple and peripheral nuclei display chromatin with a characteristic distribution and a nucleolus, which when it is visible, has a ratio nucleus / nucleolus that corresponds to 2.5:1.0. In the spaces between muscle fibers, there is a supporting tissue called endomysium. In many cases, the fibroblasts are visible, as well the delicate collagen fibrils that are distinguishable because the characteristic structure of transverse bands. In some sections, it happens small and continuous capillaries surrounded by endomysial support tissue.
By using the transmission electron microscopy, it is possible to study the mitochondrion behavior. One of the organelles that allows one to diagnose the state of metabolic activity of the cell. Mitochondrion often have a defined distribution according to cell type. They are located very close to structures that require adenosine triphosphate to work properly. The muscle contraction depends on the energy transfer from the mitochondrion. The close association between mitochondrion and sarcomeres minimizes the diffusion distance and facilitates the conversion of chemical energy into mechanical energy.
SHINDO et al (7) studied the histomorphometric and functional changes of denervated laryngeal muscles in 14 adult dogs, performing surgical removal of 2.5 cm from the right recurrent laryngeal nerve. Atrophy was observed in around three months, and after this period, it was testified the reinnervation of muscles.
KAWAKITA et al (8) promoted the unilateral denervation of the interarytenoidal muscle in Cavia porcellus, sectioning the SNL and the RLN. Five weeks after the denervation, all interarytenoidal muscle was recovered, confirming that, although the muscle has a bilateral innervation, it is neurologically supplied as a whole.
There are few studies in this field and it was not possible to find studies on the effects of unilateral denervation, followed chronologically, which had been conducted on pigs.
This study proposes to verify which muscular structures of the vocal folds, undergo through changes, when there is denervation with circulatory maintenance; verify the possibility of reinnervation of a mechanically denervated vocal fold, keeping the contralateral fold neurologically supplied; verify if, with muscle recovery, for how long after denervation, it is possible to observe the ultrastructural demonstrations, main indicators of structural restoration.
METHODThe use of animal vocal folds for research occurs because of the ethical impossibility of working with humane vocal folds. Besides, for studies in electronic microscopy, the item must be collected in vivo, before the occurance of autolysis, which begins, according RHODIN (1954) apud SANTANDER (9), three minutes after the blood circulation suspension.
It was used the commercial breed pigs (Sus scrofa domestica), with 4 to 12 weeks old. The animals had an average initial weight at the time of denervation (Phase I - First Phase) of 24.66 kgf. The animals had an average final weight at the time of biopsy of 57.2 kgf for operated animals 45 days after the denervation; 97.8 kgf for the animals after 90 days, and 149.8 kgf for animals treated after 180 days.
The animals were divided into three groups, chosen randomly. All of them underwent to the denervation of the right recurrent laryngeal nerve, with surgical removal of a segment with three centimeters. The chosen of the right recurrent laryngeal nerve is based on the finding that the left side goes through the mediastinum and may change in the organs of this area during the animal's life.
One group, consisting of 5 animals (two castrated males, one male not castrated and two females) was kept for 45 days after the denervation (Group I); another group of 5 pigs (two castrated males, a male not castrated and two females) was kept for 90 days after the denervation (Group II), and a third group, also with 5 pigs (two castrated males, one male not castrated and two females) was kept for 180 days after the denervation (Group III). The three non-castrated pigs were included as morphologic control of vocal muscle under the influence of testicular hormones.
The study was divided into two phases. In the first phase of the denervation, it was performed a frontal cervicotomy for surgical removal of a segment of three centimeters of the right recurrent laryngeal nerve, next to the cervical trachea. In the second phase, it was developed three procedures: biopsy, laryngeal resection and the death of the animal. Carried out at 45, 90 and 180 days after the end of the denervation, it was proceeded another cervicotomy to have a biopsy of the vocal muscles in its right and left fold.
Fragments of the vocal muscle were collected and immersed in fixative "S" 2% to perform the transmission electron microscopy. Subsequently, the larynx was removed and fixed in formaldehyde 10%. As the larynx was removed, the animals were immolated with an injection of KCl into the jugular vein. All animals were weighed with the primary purpose of preparing the suitable anesthetic.
Denervation
In this stage, all animals received an IM application of flunixin meglumine (Flumegam® 50mg), with an estimated 1.1 mg / kgf. After the intervention, they received a dose of antibiotic (Pentabiotic®), with prophylactic purposes. Flunixin meglumine is a non-narcotic analgesic, non-steroidal drugs (NSD) with anti-inflammatory properties, analgesic and antipyretic.
Transmission Electron Microscopy (TEM)
Fixing: the material that had a biopsy was immediately placed in fixative solution containing 25% of glutaraldehyde in Millonig buffer (Millonig (10) - 2X Concentrated pH 7.3 - more H2O Milli - Q® at a normal temperature for 60 minutes. Subsequently, the samples were subjected to three washes in Millonig isotonic buffer, for thirty minutes each. Post-fixation in osmium tetroxide: For the post-fixation with osmium tetroxide, the material remained submerged during 45min, and after this time it underwent three washes in 0.85% saline solution for thirty minutes each. Contrast: the material was contrasted, en bloc with uranyl acetate in 2% aqueous solution for 30min, followed by three washes in saline solution at 0.85% during 30min on each fase. Dehydration: it was processed in alcoholic series of 30%, 50%, 70%, 95% and 100%. All fases lasted 10 minutes, except for the last two that lasted 30 minutes. Pre-soak, soak, including: pre-soaking of the material (Figure 06) was performed in a mixture of Embed 812 (Epon substitute) with pure acetone. The whole process of pre-soaking was performed in an equipment called Rotator (Roto-torque, by Cole-Parmer Instrument Company), to ensure a circular, regular and continuous movement, to infiltrate the resin by all sides of the sample. The soaking was made in pure Embed 812 (Epon substitute) for 24 hours at the Rotator. For inclusion, it was used plastic molds and polymerization was carried out in a sterilizer with constant temperature of 60oC for 72 hours. The semislender sections were made on ultramicrotome (Leica Ultracut UCT) being used a razor diamond Drukker type histo with trough 8mm (Figure 6d) with a thickness of 800nm and stained in an 1% aqueous blue toluidine with the proportion of 1:1, 0.065% sodium tetraborate and in 0.1% basic fuchsin. To final contrast of extremely slender sections on canvas support, it was used lead citrate (REYNOLDS (11)). To the ultrastructural study, it was used a Transmission Electron Microscope Philips model EM208S.
The samples, in copper support screens with 300 mesh, were evaluated and documented with increases of 4,000, 6,000, 8,000, 10,000 and 13,000 and 40,000 times. During the observation, it was selected areas to record electromicrographs in Kodak® negative model So603, with dimensions of 6.5 cm to 9.0 cm.
Quantitative and qualitative analysis of the ultrastructure of the vocal muscle
The analysis procedures were performed with reversion to the positive, of the negative electromicrographs. It was decided for an increase of 10,000 times to perform the ultrastructural measures. These measurements were performed with the program Carnoy, developed by the Lab of Plant Systematics of the Katholieke Universiteit Leuven in Belgium (available at http://www.carnoy.org/).
It was produced 247 electromicrographs to evaluate the vocal muscle denervated and vocal muscle control (innervated), in groups that underwent to biopsies in 45 days, 90 days and 180 days after the surgery that removed a segment of the right recurrent laryngeal nerve. It was proceeded 1027 measures of the smallest mitochondrion diameters to evaluate the responses to the mechanical denervation. In the group the vocal fold had biopsy, in ninety days after denervation, in not castrated animals, just few mitochondrion were found to measurement. It was conducted a description for each electromicrographs in denervated groups and control groups, and then, all the morphologies presented were evaluated.
Statistical Analysis
Statistical analysis was performed by the Master Brasílio Ricardo Cirillo da Silva, professor of statistics of courses Undergraduate and Graduate Studies at the Catholic University of Rio Grande do Sul - PUCRS, Centro Universitário Ritter dos Reis - UniRitter and Instituto Porto Alegre da Rede Metodista de Educação - IPA, in Porto Alegre, Rio Grande do Sul. Analyses were performed with the aid of SPSS program (Statistic Package for Social Sciences) version 11 for Windows. Analysis of variance, ANOVA was performed to compare the muscles of the left vocal folds (LVF) and right vocal folds (RVF) in the three periods after the denervation (45, 90 and 180 days). The t-test, of student, was conducted to compare two groups of muscles of the vocal folds.
Bioethics
Following the Post-Graduate Health Sciences guidelines, the project was submitted to the Ethics in Research Committee, under subscription number 0426 / 02, which reviewed and approved it on June 21, 2002.
RESULTUltrastructure of the vocal muscle
The electromicrographs of the investigated material under transmission electron microscopy were classified into three groups, using as a criterion the time elapsed since the mechanical denervation until the biopsy day.
Control Units
The left vocal folds that were kept innervated, although undergoing to biopsy, according to the determined time for the different groups, presented the same characteristics between each other and also corresponding to the ultrastructure expected for a normal skeletal muscle. It was found eccentric nuclei and organized with chromatin, presenting dense granular areas along the periphery and surrounding the nucleolus (Figure 4). The sarcomeres are regular and present well-defined Z grooves and bands I, A and H. The cisterns of the sarcoplasmic reticulum, as well the triads, appear intact. Along with the cisterns of the reticules and mitochondrion, it is found plenty of glycogen granules (Figures 5 and 6). In transverse sections, the myofibrils fields surrounded by the sarcoplasmic reticulum are clear- old COHNHEIM fields (Figures 7 and 8). The not castrated animals that also underwent to biopsy in periods of 45, 90 and 180 days after the denervation of the right vocal fold, also displayed a normal ultrastructure in the vocal fold muscle which was kept with the recurrent laryngeal nerve, that is, the left vocal fold.
Tested units
The right vocal folds - tested units, mechanically denervated, also underwent to biopsy to achieve the vocal muscle, at 45, 90 and 180 days after the operation, defined as Group I, II and III.
Group I (45 days after the denervation)
The group I underwent to a biopsy forty-five days after the operation of muscular denervation, and displayed miofibrillar disorganization that disaggregate the sarcomeric architecture, leaving uncertain anisotropic bands and isotropic bands (Figure 9). In many samples, the Z lines presented to be vestigial. Mitochondrion were apparently altered compared to control, presenting reduced sizes, and fewer internal content with rare mitochondrial cristae (Figures 10 and 11). The symmetric organization between actin and myosin appears to be absent (Figure 12).
The nuclei appear morphologically normal (Figures 13). The structures of support tissue did not suffer visible changes. The collagen present in connective tissue was well preserved (Figure 14).
Group II (90 days after the denervation)
Group II, underwento to a biopsy ninety days after the operation of muscle denervation, displayed disorganized sarcomeres or presented different orientations of the expected relationships between the Z lines. The sarcoplasmic reticulum appears in an abundant way. Mitochondrion appeared reduced in size and number (Figures 15 and 16). The miofibril dissolution favoured the outbreak of electrolucid zones (Figure 17).
Disorganization does not presented itself in the same way in different materials of a same group. The disorganization degrees are changeable thoughout the vocal muscle.
Group III (180 days after the denervation)
Group III, underwento to a biopsy one hundred and eighty days after the operation of muscle denervation, it displayed regular sarcomeres with recognizable isotropic and anisotropic bands. Mitochondrion, with regular sizes and numbers when compared to controls, presented themselves aligned between the sarcomeres. The mitochondrial cristae are visible and abundant (Figures 18 and 19). Glycogen appears in large quantity near the mitochondrion and the sarcoplasmic reticulum (Figure 20 and 21).
Descriptive statistics were performed through the average, standard deviation, minimum and maximum. The results are displayed in Table 1.
Comparison of mitochondrial diameters between the different control groups obtained the following results, as displayed in Tables 2 and 3.
Comparison of mitochondrial diameters between the different groups operated for mechanical denervation of the vocal fold obtained the following results (Table 4 and 5).
The T test, the student, was performed to compare the diameters of the two mitochondrial muscle groups of the vocal folds, control versus denervated, presented the following results (Table 6).
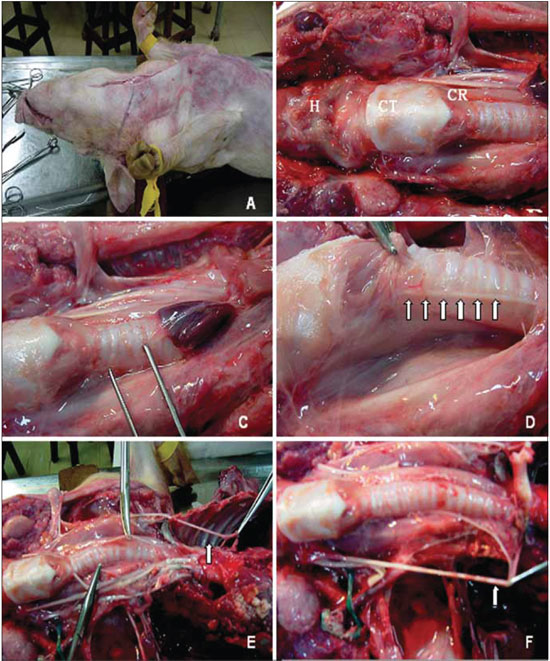
Figure 1. Anatomical aspects of reference in the ventral cervical area of the pig. The mediossagital incision in the cervical-ventral to access the content. B, points of reference: H, hyoid bone, TC, thyroid cartilage; CR, cricoid cartilage. C, the clamp displays the area near the trachea, where the recurrent laryngeal nerve is sought. D, right recurrent laryngeal nerve (arrows). And the picture shows the left recurrent laryngeal nerve originating from the mediastinum, and coursing beneath the aortic arch (arrow). F, the right recurrent laryngeal nerve cruises under the right subclavian artery (arrow).

Figure 2. Normal Ultrastructure of vocal muscle of the pig (Sus scrofa domestica). The ultrastructure of the normal skeletal muscle fiber in swines, displays the isotropic bands (I) with the presence of the Z line and anisotropic bands (A) with the presence of the central H band, crossed the M line; B, mitochondria occupy the intersarcoméricos areas (mit). C, in mammals, the triads (arrows) are located at the junctions of the A and I bands. D, between the myofibrils fields, it is often a large amount of glycogen granules (arrow heads). Increases: 10000 times in images A and D; 13000 times in images B and C.
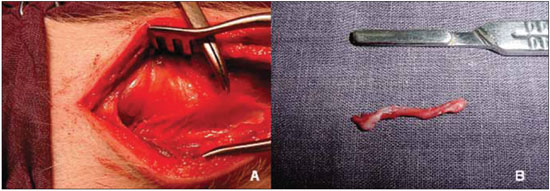
Figure 3. Denervation of the right vocal fold. A, visualization and clamping of the recurrent laryngeal nerve. B, segment excised laryngeal nerve.

Figure 4. Left vocal fold muscle. Regular organization of sarcomeres. Two nuclei well organized in its periphery (arrows). Abundant number of mitochondria (mit), linearly ordered. Increase: 4000 times. SR

Figure 5. Left vocal fold muscle. Sarcomeres well visible and well organized. Lines sorted with abundant number of mitochondria. Glycogen (gly), following in the RS areas. Increase: 4000 times. SR

Figure 6. Left vocal fold muscle. Well-organized sarcomeres. Abundant number of mitochondria (mit) and glycogen granules (gly). SR cisterns in transverse section. Increase: 13000 times.
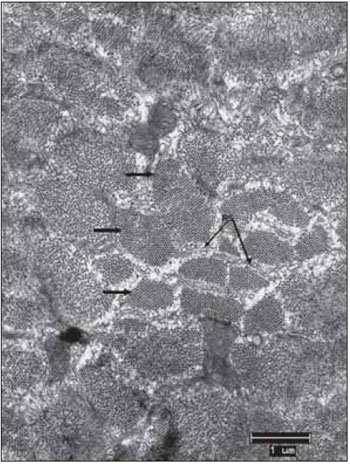
Figure 7. Left vocal fold muscle. Transverse section of the miofilaments (arrows). Beams perfectly organized and separated by sarcoplasmic reticulum - SR. Increase: 10.000 times

Figure 8. Left vocal fold muscle. Detail of miofibrils in transverse section, displaying crystalline arrangement of myosin and actin (arrows). The sarcoplasmic reticulum (SR) involves the miofibrillar fields (Cohnheim fields). At the top, two mitochondria (mit) showing ridges well developed. Increase: 40000 times.
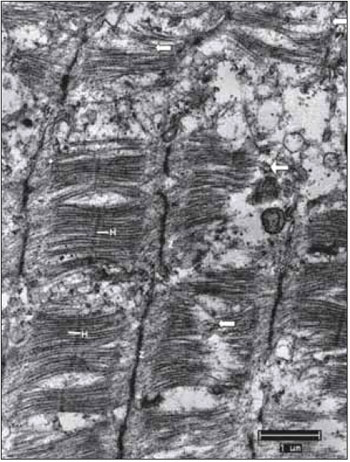
Figure 9. Vocal fold muscle after 45 days of denervation. Strong breakdown of miofibrils (arrows). Regular Z lines. H bands barely visible. Increase: 10000 times.
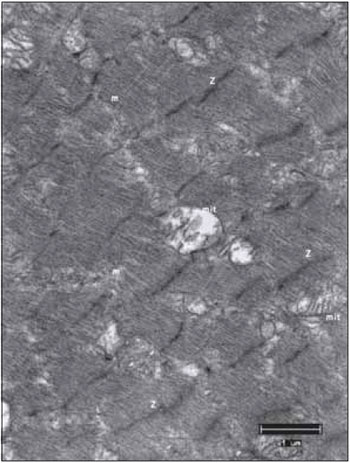
Figure 10. Right vocal fold muscle, after 45 days of mechanical denervation. Mitochondria in small numbers, with rare mitochondrial cristae compared to a control material. mit, mitochondria; Z, Z lines; mf, miofibrils. Increase: 10000 times.
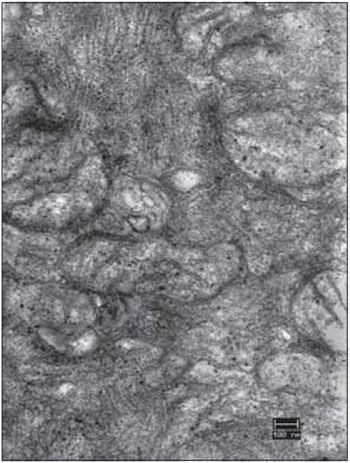
FFigure 11. Detail of the right vocal fold muscle, after 45 days of denervation. An electromicrograph presents the translucent mitochondria (mit) due to the loss of cristae and matrix. Increase: 40000 times.
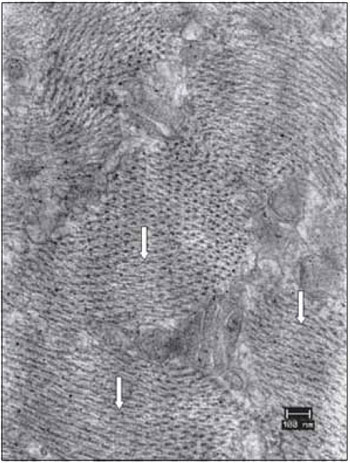
Figure 12. Details of the vocal muscle after 45 days of denervation. Miofibrils in transverse section had lost the symmetrical organization (arrows). Increase: 40000 times.
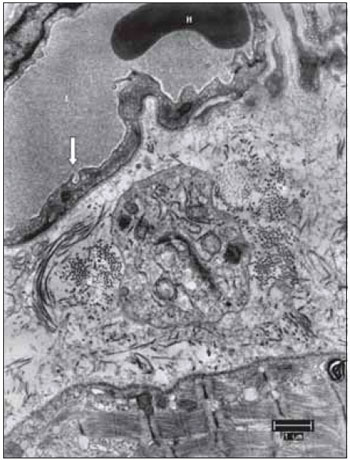
Figure 13. From top to bottom, blood capillary, supporting tissue and muscle fiber. This image allows to suggest that several components of the vocal muscle, do not suffer significant changes, such as the erythrocyte in light of the capillary, formed by endothelial cell display liquid the captivation function through the bladder pinocytotic (the arrow indicates pinocytosis of the gall bladder, characteristic of non-fenestrated capillaries). L, capillary lumen; H, erythrocyte; Co, collagen. Increase: 6300 times.
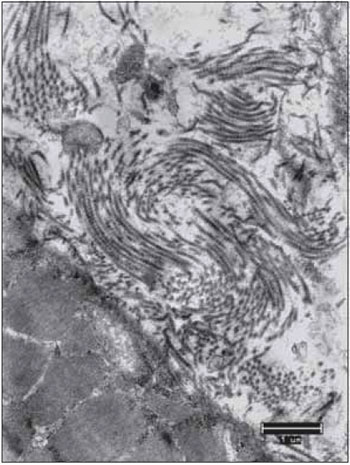
Figure 14. The collagen fibers keep the integrity in all periods after denervation. Increase: 10000 times.

Figure 15. Vocal fold muscle 90 days after the denervation. Complete miofibrillar disarray with some electrocompact mitochondria (mit). Increase: 10000 times.
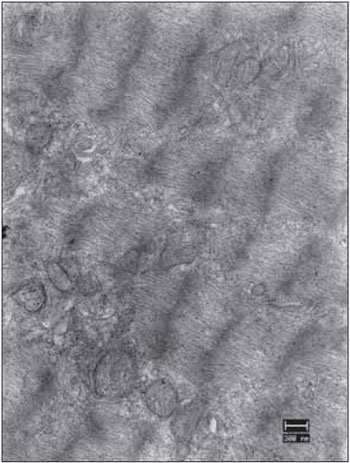
Figure 16. Vocal fold muscles 90 days after the denervation. Complete miofibrillar disarray with some mitochondria presenting few cristae (mit). Increase: 13000 times.
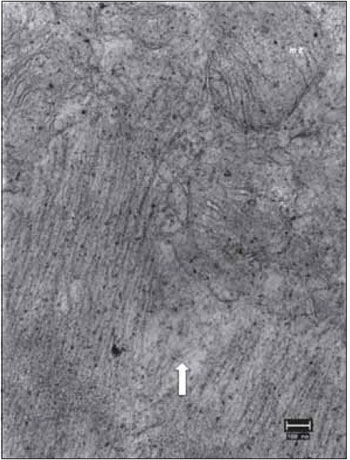
Figure 17. Vocal fold muscle 90 days after the denervation. Detail of miofibrillar disorganization (arrow) and mitochondria with few cristae (mit). Increase: 40000 times. Dense granules by osmic precipitation.

Figure 18. Right vocal fold muscle 180 days after the of mechanical denervation. Regular sarcomeres. Lines mitochondrial occupying intersarcomeric spaces. Mitochondria with volume and number expected for a normal fiber. Mitochondrial cristae developed. Normal sarcoplasmic reticulum. Increase: 10000 times.

Figure 19. Right vocal fold muscle 180 days after the mechanical denervation. Mitochondria occupy regular lines between the sarcomeres (mit); the sarcomeres present Z perfectly identifiable, clear bands and dark bands. In the center of the dark bands is easily identified the H band. Increase: 10000 times.

Figure 20. Vocal muscle of the right vocal fold 180 days after the mechanical denervation. The sarcomeres are regular and structurally normal. There is great amount of glycogen granules and mitochondria between the sarcomeric units. Increase: 10000 times.

Figure 21. Vocal muscle of the right vocal fold 180 days after the mechanical denervation. Detail of the accumulation of glycogen granules around the mitochondria. Increase: 40000 times.
Ultrastructure of the vocal muscle mechanically denervated
In the investigation of the vocal muscle of Sus scrofa domestica, with 45 days after denervation, was possible to observe that the differences are meaningful on what concerns the average mitochondrion diameter
(t = 8.34, p = 0.000 in male vocal folds, and t = 3.34,
p = 0.001, in female vocal folds). The overall average diameter of 0.48 µm in control units, and 0.39 µm tested units - right vocal muscle. In muscle with 90 days of denervation, the differences are meaningful regarding for the average mitochondrion diameter (t = 4.91, p = 0.000 in male vocal folds, and t = 7.23, p = 0.000, in female vocal folds). The overall average diameter was 0.45 µm in control units, and 0.31 µm tested units - right vocal muscle. In muscle with 180 days of denervation, the differences are not meaningful concerning to the average mitochondrion diameter (t = 1.60, p = 0. 111, in male folds and t = -1.19, p = 0.234 in female vocal folds). The overall average diameter was 0.41 µm in control units and 0.40 µm tested units - right vocal muscle (Table 3).
The ultrastructural findings in denervated muscle fibers, after 45 and 90 days of denervation, displayed a clear response to miofibrillar disorganization associated to very sharp alterations in the mitochondrion. It was observed a decrease in size (t = 4.73, p = 0.000) of mitochondria, and also a reduced number, easily verifiable by counting the mitochondrion observed in the images of control units compared to the tested units. The mitochondria also presented a considerable loss of their mitochondrial cristae, being electrocompact, by leaving electrotransparent spaces in the electromicrographs images. Mitochondrion presented themselves as the most sensitive structures to denervation.
It was also observed that the disarrangement does not appear with the same pattern in all electromicrographs of the same vocal muscle denervated, allowing to infer that the disorder is progressive - resembling a mass that first loses heat in not so deep areas, although there is no sufficient data to assert that the process of muscle disintegration of the cytoarchitecture evolves by the same method.
It was not identified cases of necrosis or phagocytosis processes, which were expected since there was no interruption in the nutritious of the muscle (the circulatory process was kept). Denervation, as it is already known, only disabled the motor activity of the vocal fold. The blood vessels and supporting tissue - endomysium - were apparently intact. The collagen did not displayed signs of change either.
As the mitochondrion displayed to be the ones that respond clearly to the process of denervation, the efforts were to evaluate these structures.
SHINDO et al (7) evaluated morphological and physiological changes in denervated laryngeal muscles in 14 dogs in which suffered a excision of 2.5 cm from the right RLN, finding muscle atrophy after 90 days of the denervation. Also in our study with pigs, the units tested with ninety days after the denervation, the picture displayed disorganized sarcomeres, electrolucid areas, due to loss of mitochondrial cristae and miofibrils.
In samples evaluated in one hundred and eighty days after the denervation, were found reorganized sarcomeres, mitochondrion with abundant ridges, mitochondrial volume and number recovered, abundant glycogen near the sarcoplasmic reticulum. It was not observed the same recovery pattern in the same vocal muscle, making believable that the reorganization is also gradual. These findings agree with the work of SHINDO et al (7) in which is also noticed that the recovery of denervated laryngeal muscles, after 90 days.
The choice of transmission electron microscopy to follow up the structural behavior of the denervated muscle, assured reliable results and an investigation into a reasonably short time. Recognizing that in humanes there are situations in which there is a unilateral paralysis of reinnervation in a period of six to twelve months, then it was searched in pigs, a model that is recognized by the scientific community as the closest to our species - in terms of metabolic, immunological and morphological systems.
The fact is, in six months there is a recovery of the muscle that had suffered the loss of the recurrent laryngeal nerve, which makes possible to assume that there was a reinnervation that emerged from contralateral or even from the superior laryngeal nerve. Innervation pattern of the porcine larynx, although generally repeats what is found in human beings, it needs to be thoroughly investigated to continue this research.
The results achieved in this work must be understood with some caution. Its conclusions are understood in the environment in which they were collected. However, it was important because evidenced new realities that can be studied in the future, as much as in theory as in experiments.
CONCLUSIONThe findings in the ultrastructure of the vocal muscles after the mechanical denervation operation of the right vocal fold of the animals, keeping innervated the left vocal cord, suggest that the muscle reinnervation, so that, the mitochondrion in the muscles presented themselves as structures more susceptible to the condition denervated, followed by the cytoarchitecture of the miofibrils. The findings in the ultrastructure of the vocal muscles, which suggest the reinnervation were observed in studies conducted on 180 days after denervation.
REFERENCES1. Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniat. 1974, 26:89-94.
2. Alajmo E, Salimbeni C. The vocal muscle. Arch Ital Anat Embriol. 1983, 88(1):15-24.
3. Stavroulaki P, Birchall M. Comparative study of the laryngeal innervation in humans and animals employed in laryngeal transplantation research. Department of Otolaryngology-Head and Neck Surgery, Northwestern University Medical School, Chicago, Illinois, USA. J Laryngol Otol. 2001, 115(4):257-266.
4. Kurita S, Nagata K and Hirano M. A Comparative Study of the Layer Structure of the Vocal Fold in Bless DM and Abbs JH. San Diego: College Hill, 1983.
5. Gorti GK, Birchall Ma, Haverson K, Macchiarini P, Bailey M. A preclinical Model for Laryngeal Transplantation: Anatomy and Mucosal Imunology of the Porcine Larynx. Transplantation. 1999, 68(11):1638-42.
6. Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2001, 110(12):1120-1125.
7. Shindo ML, Herzon GD, Hanson DG, Cain DJ, Sahgal V. Effects of denervation on laryngeal muscles: a canine model. Laryngoscope. 1992, 102(6):663-669.
8. Kawakita S, Aibara R, Kawamura Y, Yumoto E, Desaki J. Motor innervation of the guinea pig interarytenoid muscle: reinnervation process following unilateral denervation. Laryngoscope. 1998, 108(3):398-402.
9. Rhodin J. Correlation of ultrastructural organization and function in normal and experimentally changed proximal tubule cells of the mouse kidney. In Santander RG. Tecnicas de Microscopia Electronica em Biologia. Madri: Aguilar, 1968:11.
10. Millonig G. Advantages of phosphate buffer for OsO4 solutions in fixation. J Appl Phys. 1961, 32:1637.
11. Reynolds ES. The use of lead citrate at high pH as na electron opaque stain in electron microscopy. J Cell Biology. 1963, 17:208-212.
1. Master in Ciencias Otorhinolaryngology, UNIFESP. Associate Professor of Anatomy ULBRA.
2. Full Professor in Otorhinolaryngology at UNIFESP-EPM. Professor of Otorhinolaryngology, UNIFESP-EPM.
3. Doctor of Science from the University of São Paulo. Associate Professor, Department of Morphological Sciences, UFRGS.
4. Degree in Veterinary Medicine. Veterinary Medical Veterinary Hospital de Clinicas, UFRGS.
Instituition: Universidade Luterana do Brasil. Canoas / RS - Brasil. Mail Address: Henrique Zaquia Leão - Avenida Farroupilha 8001, Bairro São Luís. Canoas / RS - Brazil - ZIP CODE: 92425-900 - Telephone: (+55 51) 9973-3356 - E-mail: anato.leao@gmail.com. Article received in September 13, 2009. Article approved in March 6, 2010.