INTRODUCTIONOsteoblastoma is a rare benign tumor of the bone, usually affecting the vertebrae and long tubular bones (3-4). Its occurrence in the craniofacial region is extremely rare, especially in the nasal and paranasal fields (3,4). Nasal cavity and maxillary sinus involvement are rare sites for these lesions (3-10). Pain and swelling of the affected area are typical characteristics of benign osteoblastoma of facial bones. However, the lesion can be discovered in routine clinical examination while not presenting any signs or causing any symptoms (10,11). Computed tomography (CT) has had a crucial role in radiographic evaluation of this disease and it has strongly contributed for the differential diagnosis, management and surgical planning of osteoblastoma (5). In this article we report an atypical case of osteoblastoma of the maxillary sinus with involvement of the hard palate and show the importance of CT features in differential diagnosis of various FOL.
CASE REPORT A 40 year-old male was referred to our University, with asymptomatic facial swelling and nasal obstruction. The patient reported that the lesion had been present for at least 8 years and has been showing slow but progressive enlargement. He also reported difficulties in breathing and swallowing. Facial examination revealed expansion of the right face and involvement of the maxilla and nasal structures (Figures 1A and 1B). Dental and medical histories were uneventful. Intra-oral examination revealed mild painless swelling in the maxillary right vestibule. Conventional plain radiography (posterior-anterior (PA), lateral skull, and Water´s views) revealed a large mixed-type radiolucent and radiopaque lesion with undefined boundaries, with involvement of nasal fossa, paranasal sinuses, and hard palate (Figures 2A-C). CT showed massive involvement of the third medium of the face with heterogeneous lesions extending from hard palate to the orbital roof, filling the maxillary right sinus up to the nasopharynx region (Figures 3A-D). Based on CT images, an incisional biopsy was performed and fragments of bone and soft tissue were removed. Histopathological findings showed large osteoblast-like cells, with eosinophific cytoplasm and plumb nuclei sometimes surrounded by clusters of osteoid substance. The collagenous stroma contained osteoclast multinucleated cells and loosely aggregated fibroblast-like cells (Figure 4A and 4B). Final diagnosis of osteoblastoma was established. Thus, a multidisciplinary approach was taken for surgical and clinical treatment, given the lesion's massive size. The proposed surgical plan included partial removal of the lesion in order to improve the patient's breathing and swallowing difficulties. The patient was submitted to oral approach for partial removal of the tumor. A second surgical intervention was proposed to repair a facial defect due to the first intervention. However, the patient refused to be submitted to another surgery. He has been followed in our service and no tumor growth has been observed to date in follow-up for two years.
DISCUSSIONSome cases of fibrous osseous lesions with nasal involvement are clearly reported (1,2). These cases had similar characteristics to our case demonstrated. Only few cases of osteoblastoma with nasal cavity and maxillary sinus involvement have been reported in the literature (3-10). It has most commonly been found in patients younger than 30 years of age with a peak incidence in the second decade of life ranging from 3 to 78 years. Pain and swelling of the affected area has been typical features of benign osteoblastoma of facial bones. However, the lesion can be found during routine clinical examination while not presenting any sign or causing any symptoms. Radiographic findings have consisted of well or poorly radiolucent defined area with signs of mineralization (10,11). In our case, the patient reported no pain and reported that the lesion had been present for at least 8 years with slow growth.
Radiological features of FOL like fibrous dysplasia, cementifying dysplasia, cemento-ossifying fibroma are classically defined fusiform enlargements (especially in the mandible) or generalized enlargements of the upper jaw. If sufficiently large, it may lead to obliteration of the lumen of the maxillary antrum. Although these features are generally visible on conventional radiographs, CT scans may be required for a more comprehensive evaluation, which is especially useful for confirming diagnosis and assessing the extent of FOL in the craniofacial skeleton (12). An incisional biopsy was performed for final diagnosis. Histopathological revealed that the tumor is composed of large osteoblats-like cells, showing eosinophific cytoplasm and plumb nuclei sometimes surround clusters of osteoid substance. The collagenous stroma contains osteoclast multinucleated cells and loosely aggregated fibroblast-like cells. Some features of osteoblastoma were seen in the lamina.
Differential diagnoses in the CT findings of FOL and osteoblastoma include lesions such as osteoid osteoma, fibrous dysplasia, central ossifying fibroma, and malignancies as low grade osteossarcoma (1,5,12). In our case, CT scan patterns were compatible with a wide range of lesions, being inconclusive for clinical hypotheses with similar features observed in fibrous dysplasia reports (1). Since some features found in the CT scan were heterogeneous, with existence of hyperdense and hypodense areas with different degrees of mineralization, tomographic examination was not compatible with cited lesions. Based on the CT scan characteristics we decided to remove both soft and hard tissue for histopathological evaluation, searching for a more representative sample of fibrous and hard tissues.
Central ossifying fibroma and fibrous dysplasia in some cases have similar histological and radiological features. Both entities may show different range of densities due to different proportions of fibrous and osseous components similar to osteoblastoma (2,3,13). Therefore, the borders of the lesion remain the major radiographic discriminator between fibrous dysplasia and ossifying fibroma. In our case the lesion have well demarcated margins on CT, therefore sharpness of the borders cannot be regarded as a differentiating feature. Radiographically, they may be indistinguishable, since ossifying fibroma usually presents well-delineated radiolucency with varying amounts of radiopaque material. The difficulty arises when the tumor shows marked osteoblastic activity. In ossifying fibroma, the intertrabecular spaces should not reveal the large plump osteoblasts seen in osteblastoma. CT scan images of our case showed a massive involvement of the third medium of the face with heterogeneous lesions extending from hard palate to orbital roof, filling the maxillary right sinus up to the rhinopharyngeal region. High degree of structure involvement was seen.
Cementoblastoma is another type of fibrous osseous lesion to be considered as differential diagnosis of osteoblastoma in radiographic findings. The key feature to differentiate it from osteoblastoma is the merging of the lesion and the radicular surface of the tooth (10,14). In the present case, no contact of the lesion with tooth was observed and, therefore, hypothesis of cementoblastoma was discarded.
The lesion that most intimately relates to osteoblastoma is the osteoma osteoid. Some authors believe that both types are probably clinical and anatomical variants of the same osseous tumor of osteblastic origin (10). Osteoma osteoid can also be confined to the nasal cavity. Osteomas can be very dense well-defined tumors, if classified as compact type. Cancellous osteomas may contain areas of low density with thick bony trabecula, due to their fibrovascular stroma. Small lesions may be less obvious on plain radiographs, but they are easily depicted on CT, showing non-aggressive radiological features. The peripheral parts of the lesion in our case showed mixed areas being very dense in the inferior portion. These features were different from typical patterns of osteoma, being more consistent with fibrous displasia or other FOL such as ossifying fibroma. We discarded osteoid osteoma, since we did not observe factors that would characterize it, such as size, sclerotic area nidus at the center of the lesion, pain and history of trauma or infection in the region.
Given the existence of inconclusive histopathological results, conventional radiographic features, and CT images, the possibility of malignancy should be considered. Osteossarcoma is so far the most important condition one should consider in the differential diagnosis of osteoblastoma. The distinction between a conventional osteoblastoma and a low grade osteossarcoma is extremely difficult (15-16). Radiographically, osteossarcoma is not as well-defined, and its histopathology contains atypical osteoblasts, with malignant bone formation, capable of presenting sarcomatous stroma as well anaplastic cartilage. These features differentiate it from osteblastoma(10). Our case showed radiographic findings such as hyperdensity and heterogeneous features similar to osteossarcoma, but the long term history (8 years) excluded this hypothesis. Low grade osteossarcoma grows more rapidly and tends to cause inferior lip paresthesia when compromising the jaw, as a result of impairment of the inferior alveolar nerve(10).
CT is also superior in demonstrating remodeling and erosion of the adjacent bony structures, a remarkable feature in cases of benign bone tumors. In addition, surrounding soft tissue edema and muscle wasting could be evaluated as well. Although these features contributed to the evaluation and surgical planning of osteoblastomas, CT scans did not contribute for the final diagnosis but important tool for differential diagnosis. Critical evaluation of CT images is an important step before performing biopsies. Based on CT images, one can select specific areas of soft and hard tissues with substantial stroma. This procedure can result in a more effective tissue analysis, giving more information about the real nature of the lesion. This is especially important in controversial cases, when radiographic and clinical characteristics are inconclusive. In our case, a CT evaluation was used as guidance for incisional biopsy of the lesion.
In accordance to SMITH (12), we have been impressed with the frequently overlapping and seemingly close relationship of these benign fibro-osseous lesions of the nasal and paranasal structures, ranging from fibrous dysplasia to (cemental) ossifying fibroma to osteoblastoma (osteoid osteoma) and cementoblastoma. In the absence of recognized origin of any of these lesions, it is possible that they are all variations of a single disorder.
We believe that surgeons must be aware in their practice of the entity of benign osteoblastoma with maxillary sinus, hard palate, and nasal involvement. They should consider this diagnosis based on the correlation of clinical appearance, imaging, and histopathological data.
When the diagnosis of osteoblastoma is confirmed, curettage or local excision should be performed (10). We recommend vigorous curettage followed by bur ablation and copious irrigation. The treatment of choice is complete surgical excision. However, sometimes it is difficult to judge whether or not to sacrifice important anatomical structures (5). Our case demonstrated that surgical procedures are very complex and can result in deformity of the face. In cases with extreme involvement of facial structures, careful evaluation and multidisciplinary approaches are required for treatment and rehabilitation of the maxillofacial complex.
Multidisciplinary approaches are required since complex cases with high degree of structure involvement require integration not only for purposes of recreating a patent airway, but also to provide functional and aesthetical structures.
Follow-up is strongly recommended because inadequately removed tumors may recur. Nevertheless, recurrent osteoblastoma-like lesions may be a pathognomic indicator of malignancy, or at least of a malignant tendency of the tumor, justifying the poor prognosis following metastasis or local expansion (17).
The patient refused to be submitted to a new surgical intervention to correct a facial defect resulting from the first surgery. He has been followed in our service every three months. No tumor growth has been detected to date.
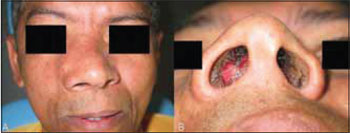
Figure 1. Facial examination: excision of the right face.
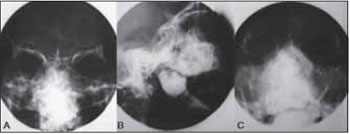
Figure 2. Conventional plain radiography of face.
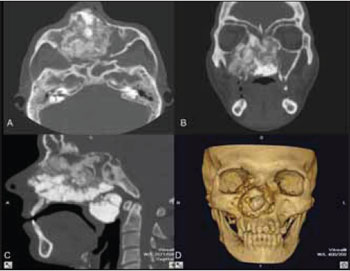
Figure 3. CT showed involvement of the third medium of the face.
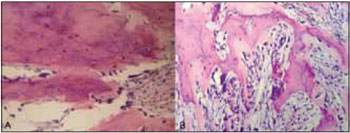
Figure 4. Histopathologic structures.
Due to their clinical and radiological similarities of others fibrous osseous lesion, the osteoblastoma should be carefully evaluated and histhopathological findings contribute to the final diagnosis. Multidisciplinary approaches should be considerated for this case. CT scans have an important role in cases like this, contributed to differential diagnosis and this method can easily demonstrate extent of the tumor, tumor delineation, characterized as peripheral thin bony shell.
BIBLIOGRAFICAL REFERENCES1. Atilla S, Pabuscu Y, Celasun B, Gerek M, Pocan S. Radiology of an atypical fibro-osseous lesion confined to the nasal cavity. Neuradiology. 1998, 40(11):752-754.
2. Wenig BM, Tuyethoa NV, Smirniotopoulos JG, et al. Aggressive psammomatoid ossifying fibromas of the the sinonasal region. Cancer. 1995, 76(7):1155-1165.
3. Som PM, Bellot P, Blitzer A, Som ML, et al. Osteoblastoma of the ethmoid sinus, the fourth reported case. Arch Otolaryngol. 1979, 105(10):623-625.
4. Imai K, Tsujiguchi K, Toda C, Sung KC, et al. Osteoblastoma of the nasal cavity invading the anterior skull base in a young child. J Neurosurg. 1997, 87(4):625-628.
5. Velegrakis GA, Prokopakis EP, Papadakis CE, Karampekios SK, et al. Osteoblastoma of the nasal cavity arising from the perpendicular plate of the ethmoid bone. J Laryngol and Otol. 1997, 111:865-868.
6. Burguera FG, Meseguer DH, Riu MR, Rosado JP, et al. Osteoblastoma de la cavidad nasal: A propósito de um caso. An Esp Pediatr. 1997, 46:83-84.
7. Fu YS, Perzin KH. Non epithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: A clinicopathologic study II. Osseous and fibro-osseous lesions, including osteoma, fibrous dyplasia, ossifying fibroma, osteoblastoma, giant cell tumor, and osteossarcoma. Cancer. 1974, 33(5):1289-1305.
8. Ducastelle T, Dehesdin D, Hemet J, Andrieu-Guitrancourt J. Osteobastome bénin sous-périosté de la cavité nasale. Etude histologique et ultrastructurale d'une observation. Ann Pathol. 1985, 5(2):131-136.
9. Sooknundum ML, Kacker SK, Kapila K. Benign osteoblastoma of the nasal bones (a case report). J of Laryngol and Otol. 1986, 100:229-232.
10. Capelozza ALA, Dezotti MSG, Álvares LC, Fleury RN, et al. Osteoblastoma of the mandible: systematic review of the literature and report of a case. Dentomaxillofac Radiol. 2005, 34:1-8.
11. Huvos AG. Bone tumours, diagnosis, treatment and prognosis, ed 2. Philadelphia: WB Saunders 1991, 67-83.
12. Smith RA, Hansen LS, Resnick D, Chan W. Comparison of the osteoblastoma in gnathic and extragnathic sites. Oral Surg Oral Med Oral Pathol. 1982, 54(3): 285-298.
13. Nakagawa K, Takasato Y, Yoshifumi I, Yamada K. Ossifying fibroma involving the paranasal sinuses, orbit, and anterior cranial fosa: case report. Neurosurgery. 1995, 36(6):1192-1195.
14. Slootweg PJ. Cementoblastoma and osteoblastoma: a comparison of histologic features. J Oral Pathol. 1992, 21(9):385-389.
15. Mcleod RA, Dahlin DC, Beabout JW. The spectrum of osteoblastoma. AJR Am J Roentgenol. 1976, 126(2):321-355.
16. Greer RO, Berman DN. Osteoblastoma of the jaws: current concepts and differential diagnosis. J Oral Surg. 1978, 36(4):304-307.
17. Ueno H, Ariji EI, Tanaka T, Kanda S, Mori SI, et al. Imaging features of maxillary osteoblastoma and its malignat transformation. Skeletal Radiol. 1994, 23(7):509-512.
Institution: Faculdade de Odontologia da Universidade de São Paulo. São Paulo / SP - Brazil.
Mail address: Estevam Rubens Utumi - Rua Pelotas 284 - Apto. 21 - Vila Mariana - São Paulo / SP - Brazil - Zip Code: 04012-000 - Celullar phone: (+55 11). 9966-6644 - E-mail: estevamutumi@uol.com.br. Article received on December 11, 2008. Article accepted on November 13, 2009.