INTRODUCTIONThe head and neck cancer (HNC) has an incidence of two to three percent of all human neoplasms and takes the sixth place as the most frequent type of cancer worldwide. It occurs more typically in patients of the male sex between the fifth and the eighth decade of life and is less common in patients younger than 45 years old. Forty per cent of the head and neck cancer occur in the oral cavity, 25% in the larynx, 15% in the pharynx and 20% in other parts, including salivary glands. The HNC represents a significant health problem and causes almost 200,000 deaths all over the world yearly. In India, the oral spinocellular carcinoma (SCC) corresponds to approximately 50% of the new cases of cancer diagnosed and its incidence is growing in spite of the advances of prevention and new therapies (1.2). Nevertheless, the rates of morbidity and mortality have had a few improvements during the last 30 years, especially when it occurs in young patients, before the fourth and fifth decades of life (3, 4). Recent clinical observations have shown that oral cancer in young patients has a specific etiology and clinical progression in which the genetic factors prevail irrespectively of exogenous carcinogenic factors, such as smoking and alcoholism (5, 6, 7).
When there is exposure to carcinogenic agents in cells like those of the mouth and pharynx, there may be the "cancerization" of such cells whose first stage is the appearing of atypical and dysplastic cells with several genetic and molecular changes with a final result of neoplasm (7, 8). Therefore, the genetically altered cells spread and expand gradually to adjacent parts that are still without neoplasm, but are already vulnerable to cellular mutations. These regions that are normal upon clinical exam and optical macroscopy are susceptible to evolve into neoplasms, which would explain many neoplasm recurrent situations and the occurrence of a second primary tumor (9, 10). The probability for this to occur in oral cancer is of 10 to 25%, especially because many of these patients keep the prior characteristics of exposure to carcinogenic agents, like the habit of smoking and drinking alcoholic beverages, now connected to therapeutic irradiation (11).
However, the concept of evolution from normal cells at optical microscopy into neoplastic cells is not very clear yet so far. The concept that is current today is that these normal cells adjacent to the tumor would only be in stages different from the dormant cellular cycle but already with potential nuclear mutations into neoplastic cells (12, 13).
Biomolecular researches indicate that these cells have certain genes undergoing mutations that depend or not on the action of exogenous factors (14). The alterations of the genes and the expression of proteins they encode would make the cells lose the capacity to adjust their growth and cause the appearing of neoplasms (15).
One of the first genes that are more studied from this point of view is Tp53 and its expression is protein p53. When a cell is exposed to carcinogenic agents, there is an increase to the levels of protein p53 that connect to transcription factors and prevent the cell to get in phase S of the cellular cycle (16). The reversible stoppage of the cellular cycle at transition C1-S permits the DNA repairing and the establishment of the genome integrity. When the DNA alterations exceed the repairing capacity, there occurs cellular death by apoptosis and very often loss of cellular growth control. We notice evolving immature cells with a strong degree of aneuploidy, loss of heterozygosis and aberrant DNA expression (17, 18, 19, 20).
Currently, the importance of gene Tp53 would be that of the "guardian of the genome" able to control the DNA duplication quality when detecting an error at the sequence of the cellular cycle, which causes cellular apoptosis and would prevent the loss of control of cellular differentiation (16, 17).
However, there is no unanimity concerning such observations. Immunopathological studies of lesions to the mouth, pharynx and larynx did not find any statistically different alterations between the p53 altered percentage, severity of the epithelial dysplasia and neoplastic lesions (18, 19, 20, 21). An explanation to these dissentient findings is that the overexpression or lack of expression of p53 and other cellular markers may not always indicate the presence or absence of neoplasm. Another hypothesis is that the expression of p53 would be masked by gene deletion or even connected to virus such as hpv (22, 23).
A current interesting description is that the study of genetic alterations like those of p53 may be used for tumor resistance prognosis to radiotherapy and chemotherapy (24, 25, 26). Even today in the clinical practice we ascribe the tumor resistance of the treatment to the selective advantages of the neoplastic cells on normal cells for the loss of control mechanisms that regulate their growth and the cellular proliferation (27, 28). The clinical applicability of these concepts like that of obtaining morbidity and mortality predictive values of patients with oral and pharynx cancer would be a great advance (29, 30).
Unfortunately, today the therapeutic choice in patients with this type of cancer is still based on clinical staging and histological graduation of the neoplasms. Although current concepts of organs preservation connected to chemotherapy and radiotherapy, considered so far to be secondary in head and neck cancer, have gained preference for the therapies of such cancer, which decreases the surgery extension and improves the quality of life, we still do not apply the molecular biology as an instrument for the choice of the treatment modality.
Thus, the objective of this work is to describe the gene alterations, specifically the p53 expression in neoplastic lesions of the oral and pharyngeal mucosa and areas close to the tumor, that are normal upon histopathology after radiotherapy in individuals with oral and pharyngeal spinocellular carcinomas.
METHODThis work was submitted and approved without limitations by the Ethics Committee in Research under number 435/2002-CEP, of our institution. The study is clinical, prospective and a one-year follow up of twenty-four patients with oral and pharyngeal (base of the tongue) carcinoma. The criteria for inclusion of patients were: individuals without diagnostic confirmation of cancer, who had no treatment, results of the histopathological exam of the neoplasm made in our hospital, confirmed to be spinocellular carcinoma and of the mucosa close to the tumor with absence of neoplasm. The treatment chosen was associated cobalt therapy and chemotherapy and the main authors did not take part in the choice of treatment. The variables studied were age, sex, smoking, alcoholic, tumor TNM classification, region of the tumor and presence or absence of expression p53.
This study included 24 patients with oral and pharyngeal cancer (base of the tongue) aged between 43 and 95 years (mean age of 69 years old), 22 (91.7%) of the male sex and 2 (8.3%) of the female sex (Table 1).
Fifteen patients were smokers, more than a package/day, on average for more than 10 years (positive), 2 were non-smokers (negative) and 7 had quit smoking before the entering our hospital. As for the use of alcohol, ten patients were alcoholic, four were non-alcoholic (negative) and ten reported having quit drinking and smoking when they were informed they had or could have cancer. These patients had been for 4 months on average without drinking alcoholic drinks or smoking.
As for the location of the neoplasm, twelve patients had lesion on the base of the tongue (50%), five patients on the floor of the mouth, two on the right side and three on the left side, six on tonsillar pillar (3 to the right and 1 to the left), and one on retromolar area. Four patients had clinical staging T1N0M0 (16.7%), six had T2N0M0 (25%), seven had T3N2M0 (29.2%) and seven had T4N2M0 (29.2%) (Table 1).
Two initial biopsies were made on different areas, the first of which on the neoplastic lesion (A1) and the second biopsy on average one centimeter away from the neoplasm, on normal mucosa upon clinical and histopathological exam (A2). After the end of radiotherapy and chemotherapy treatment, two new biopsies were done again, one on the neoplasm or on the delimited area before starting the treatment and the other on the normal mucosa. The time interval between the first and the second biopsy was of 12 months.
Each of the tissue fragments was fixed in formalin, added to paraffin separately and colored by the hematoxylin-eosin technique. The histological analysis and histological graduation were made: slightly, moderately or well-differentiated. As regards to the degree of histological differentiation, we found 8 individuals with carcinomas moderately differentiated (Figure 1A), 14 well-differentiated (Figure 1B) and 2 slightly differentiated.
The tissue samples fragments (A1) and (A2) were cut in blocks and the detection of the tumor suppressing gene TP53 was done by means of immunohistological reaction with the monoclonal antibody anti-p53 produced in mice (DAKO Code M7001).
The results were evaluated in common optical microscopy and the positive reaction was represented by deposition of the chromogen on connection sites antigen-antibody, with provision of nuclear marking to cells that presented the antigens studied. The analysis of the expression was considered to be positive upon optical microscopy when we detected the cell nucleus with a strong brown color (Figure 1C) and negative when light brown or transparent (Figure 1D). The positive criterion of the p53 expression was given by the counting of 10 or more cells (10%), in a total of 100 cells per reticulum, by counting the cells in at least three reticula (HAMILTON and ALLAN, 1995) (33).
We performed a comparative study of the p53 expression with the variables area of the tumor, alcoholism, smoking and staging. For statistical analysis, we used the Chi-square tests, the Fischer's exact test and McNemar's test. The significance level used was of p<0.05.
RESULTSWe noticed an overexpression of protein p53 in the initial biopsy, before the treatment, in 14 of the 24 samples of adjacent normal mucosa (ANM) and in 20 of the twenty-four samples of the neoplastic lesions. After radiotherapy and/or chemotherapy treatment, out of fourteen ANM samples, with positive expression of the p53, 6 maintained the overexpressed p53 and 2 negatives. In the 20 neoplasm samples with increased p53 expression, they remained positive for seven patients, two of the cases had a negative expression of the p53. Eleven patients could not be evaluated for having died before the second immunohistochemical evaluation.
From the patients who died, one had staging T1, two had staging T2 (18.2%), six (54.6%) staging T3 and 2 staging T4 (18.2%) (p<0.05). As regards to the histological graduation of these patients, we noticed that five cases were of well-differentiated, five were moderately differentiated, and one slightly differentiated of SCC. There was a statistically significant difference for the p53 overexpression relationship, both in the tumor and the normal adjacent mucosa, with the tumor staging, but not with any degree of the neoplasm differentiation (Tables 2 and 3). An important observation is that five patients with overexpression of the p53 were aged below 50 years old. The p53 positive expression association with exogenous factors was statistically evident for smoking (p = 0.005) and not significant for the use of alcohol (p = 0.22).
DISCUSSIONThe data of our work confirmed the literature, that is, the incidence of oral and pharyngeal spinocellular carcinoma is predominant in men, older than the fifth decade of life and having smoking and alcoholism as their risk factors (1, 2). These works report that smoking separately increases the risk of cancer development in six times and alcohol in 3.5 times. When associated, such risk indicators can increase in up to 15 times the possibility to develop oral and pharyngeal cancer, and the induced malignant transformation potential by these agents is durable and the risk is dose-dependent (7, 11). In our study, such observations were not different; 90% of the patients with oral cancer were smokers and 75 to 80% were alcoholic, taking into account the individuals that reported having quit alcohol and smoking a few weeks before definitive diagnosis of cancer.
However, the observation of oral and pharyngeal spinocellular carcinoma in five patients younger than 50 years, with aggressive neoplastic clinical characteristics, shows us the establishment of the pathology of cancer in these individuals could be associated to other risk factors, such as the genetic inherited characteristics and not only induced cellular mutation by exogenous carcinogenic agents (3, 4, 5, 6). In these cases, the ideal for diagnosis and life prognosis is that we should more and more use methods that compute not only histological findings, like types and degrees of the neoplasms cellular differentiation, but also the molecular characteristics and genetic susceptibility of the oral and pharyngeal carcinoma neoplastic cells (15, 24, 25, 26). In the last two decades, the descriptions of TP53, K167 genes, amongst others, with cellular growth control functions presented new perspectives as regards to the treatment and prognosis of this type of cancer (30).
These new concepts are far interesting for us to understand whether the normal and/or dysplastic cells progression for cancerization, especially in young individuals, occur by means of carcinogenic genetic mutations alterations that could already be present and not yet visible through conventional histology (27, 28, 29). This concept that the cancer genetic theory is very timely today was what led us to research genetic changes not only in the tumor but also in the normal adjacent mucosa on the neoplasm (12). In our findings, the p53 increased expressions occurred both in the area of the tumor and in the normal mucosa. For REGESI et al. (1995) (19) and AGUIAR and ARAÚJO (1997) (20) such changes could have a value of prediction as regards to the appearing of a neoplasm or of resistance upon radiotherapy and chemotherapy. Despite not conclusive, our data seems to confirm such information because most patients who had overexpression of the p53 had large tumors and reacted negatively to the radiotherapy. In these individuals, the persistence of overexpression of the p53, both in the tumor and in the normal mucosa could have an indication of a higher resistance to radiotherapy treatment, and consequently affect the survival of patients with oral and pharyngeal cancer (30, 31).
However, such observations are not conclusive. The clinical pathological correlation we described between the size of the tumor and the p53 overexpression indicates that possible genetic and molecular changes, between them, of the p53, when detected in individuals with oral and pharyngeal carcinomas can be a bad prognosis factor, especially when in the case of the p53 expression and of other proteins in areas of the normal mucosa adjacent to the tumor (2, 20, 26). Such findings would show a possible multicentric origin of the oral and pharyngeal cancer and the examiner must make biopsies and exams in other sites close to the lesion and not only on the neoplasm; molecular lesions could be present in the epithelium adjacent to the tumor but not visible in optical microscopy (12). This meets the field cancerization theory (12), and explains the appearing of a second primary tumor and of many neoplastic recurrences (9, 10, 11). Finally, the exact molecular characteristic of the genetic susceptibility of this changed tissue field is not clear yet, but tumor suppressing genes, like Tp53 may be the key factor in the initial stages of the cancer, by opening several possibilities to predict short time better results for treatment and life prognosis.
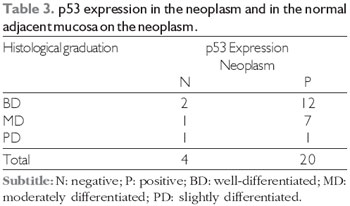
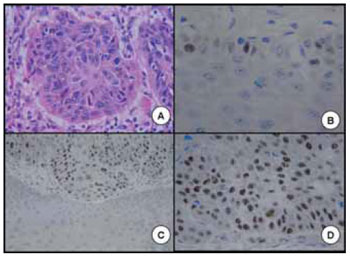
Figure 1. A. Spinocellular carcinoma moderately differentiated of pharynx. B. Spinocellular carcinoma well-differentiated of mouth. C. Positive expression of the p53 in oral spinocellular carcinoma. D. Negative expression of the p53 in oral spinocellular carcinoma.
The findings of this study show the p53 expression was increased in 20 of the 24 biopsies in the area of the neoplasm and in 14 biopsies of the normal mucosa adjacent to the tumor before beginning of the radiotherapy, and biopsy was maintained after the end of radiotherapy treatment. There was a statistically significant correlation between the increase of the p53 with the size of the neoplasm and smoking, but not with the degree of cellular differentiation.
BIBLIOGRAPHICAL REFERENCES1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen majors cancers in 1985. Int J Cancer. 1993, 54:594-606.
2. Kowalski LP, Franco EL, Torloni H, Fava AS, Andrade Sobrino J, Ramos G, Oliveira BV, Curodo MP. Lateness of diagnosis of oral and oropharyngeal carcinoma: factors related to the tumor, the patients and health factors. Oral Oncol Eur J Cancer. 1994, 30:167-73.
3. Kock WM, Lanzo M, Seurel D, Zohura KM, Sidransky. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999, 109:544-51.
4. Martin-Granizo R, Rodrigues-Campo F, Naval Z, Gonçalez FJW. Squamous cell carcinoma of the oral cavity in young people- a comprehensive literatura review. Oral Oncol. 2001, 33:401-18.
5. Mackenzie J, Ah-See K, Thakker M, Sloan P, Marau AG, Birch J, et al. Increasing incidence of oral cancer among young person: what is the aethiology? Oral Oncol. 2000, 36:387-9.
6. Schantz SP, Yu G. Head and neck cancer incidence trends in young americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaringol Head Neck Surg. 2002, 128:268-74.
7. Lewellyn CD, Johnson NW, Warnakulasurixa KA. Risk factors for squamous cell carcinoma of the oral cavity in young people-comprehensive: literature review. Oral Oncol. 2001, 37(5):401-18.
8. Rodrigo JP, Suaréz C, González MV, Lazo PS, Ramos S, Coto E, Alvarez I, García LA, Martínez JA. Variation of genetic alterations in different sites of head and neck cancer. Laryngoscope. 2001, 111:1297-01.
9. Jones AS, Phillips DE, Husband D. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995, 75:1343-53.
10. Cianfriglia FD, Gregorio DA, Manieri A. Mutiple primary tumor in patient with oral squamous cell carcinoma. Oral Oncol. 1999, 35:157-63.
11. Franco EL, Kowalski LP, Kanda LL. Risk factors for second cancers of the upper respiratory and digestive systems: a case- control study. J Clin Epidemiol. 1991, 44:615-25.
12. Califano J, Van der Riet O, Westra W, Nawwroz H, Clyman G, Prantadon S, et al. Genetic progression model for head and neck: implications for field cancerization. Cancer Res. 1996, 56:2488-92.
13. Neville BW, Day TA. Oral cancer and precancerous lesions. Cancer J Clin. 2002, 52:195-215.
14. Nagai MA, Miracca EC, Yamoto L, Kowalski LP, Bremtani RR. TP53 Mutations in upper aerodigestive squamous cell carcinomas from a group of Braziliam patients. Am J Surg. 1995, 170:492-4.
15. Coltrera MD, Zarbo RJ, Sakr WA, Gown AM. Markers for displasya of the upper aero digestive tract. Suprabasal expression of PCNA, p53 e CK 19 in alcohol-Fixed, embebeded tissue. Am J Pathol. 1992, 141:818-25.
16. Veiculoscu NE, El-Deiry WS. Biological and clinical importance of the p53 tumor suppressor gene. Clin Chem. 1996, 42:858-68.
17. Shin DM, Kim J, Ro JY, Hittelman J, Roth JA, Hong WK, Hittelman WN. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994, 54:321-6.
18. Wood MW, Medina JE, Thompson GC, Houck JR, Min KW. Accumulation of the p53, supressor gene product in oral leukoplakia. Otolaryngol Head Neck Surg. 1994, 111:758-763.
19. Regesi JA, Zarbo RJ, Regevi E, Pysanty S, Silver-Man S, Gazit D. p53, protein expression sequential biopsies of oral displasias and in situ carcinomas. J Oral Pathol Med. 1995, 24:18-22.
20. Aguiar MCF, Araújo VC. p53 protein expression in lining epithelium adjacent to oral squamous cell carcinoma. Rev Pos Grad. 1997, 4:14-9.
21. Chung KY, Mukhopadhyay T, Kim J, Casson A, Ro JY, Goepfert H, Hong WK, Roth JA. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res. 1993, 53:1676-83.
22. Naggar AKE, Lai S, Glayman GL, Mims B, Lippman SM, Coombes M, Luna MA, Lozano G. p 73, gene alterations and expression in primary oral and laryngeal squamous carcinomas. Carcinogenesis. 2001, 22:729-35.
23. Yoo GH, Washington J, Oliver J, Piechocki M, Kim H, Nora JF, Shibuya TY, Wilson DR, Ensley JF. The effects of exogenous p53 overexpression on hpv-immortalized and carcinogen transformed oral keratinocytes. Am Cancer Soc. 2002, 94:159-66.
24. Rouband-Diogene H, Fortin A, Morency R, Roy J, Monteil RA, Têtre B. Markers of radioresistance in squamous cell carcinoma of the head and neck: a clinicopathologic and imunohistochemical study. J Clin Oncol. 1997, 8:1030-8.
25. Couture C, Diogéne HR, Têtu B, Bairati I, Mury D, Allard J, Fortin A. p53 and ki-67 as markers of radioresistance in head and neck carcinoma. Am Cancer Soc. 2002, 94:713-22.
26. Gluckman JL, Pavelic ZP, Welkoborsky HJ, Mann W, Stambrook P, Gleich L, Wilson K, Righi P, Portugal LG, Mcdonald J, Biddinger, Steward D, Gartside P. Prognostic Indicators for Squamous Cell Carcinoma of the Oral Cavity: A Clinicopathologic Correlation. Laryngoscope. 1997, 107:1239-1244.
27. Brachman DG, Beckett M, Graves D, Haraf D, Vokes E, Weichselbaum RR. p53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell lines. Cancer Res. 1993, 53:3667-9.
28. Pirollo KF, Hao Z, Rait Yang YJ, Fee Wee JR, Ryan P, et al. p53 mediated sensitization of squamous carcinoma of the head and neck to radiotherapy. Oncogene. 1997, 14:1735-46.
29. Lavertu P, Adelstein DJ, Myles J, Secic M. p53 and ki-67 as outcome predictors for advanced squamous cell cancers of head and neck treated with chemoradiotherapy. Laryngoscope. 2001, 11:1878-92.
30. Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otorhinolaryngol Head Neck Surg. 2000, 122:44-51.
31. Weichselbaum RR, Kufe DW, Advani SJ, Roizman B. Molecular targeting of gene therapy and radiotherapy. Acta Oncol. 2001, 40:735-8.
32. Goepfert H. Squamous cell carcinoma of the head and neck: past progress and future promise. CA Cancer J Clin. 1998, 48:195-198.
33. Hamilton PW, Allen DC. Morfometry in histopathology. Journal of Pathology.
1 Master's Degree in surgery from the Medical College of Botucatu. Dental Surgeon.
2 Lecturer in Otorhinolaryngology. Coordinator of Discipline in PG sensus stricto.
3 Doctoral Degree. Doctor in Pathology of the Base Hospital of Bauru - SP.
4 Doctoral Degree. Doctor Professor of the Biosciences Institute of Botucatu - Unesp.
Institution: Faculdade de Medicina de Botucatu - Depto. OFT/ORL/CCP. Botucatu / SP - Brazil. Mail address: Jair Cortez Montovani - Faculdade de Medicina de Botucatu - Depto. OFT/ORL/CCP - Distrito de Rubião Júnior s/n - Botucatu / SP - Brazil - Zip code: 18618-970 - Fax: (+55 14) 3811-6256 / 6081- E-mail: montovan@fmb.unesp.br
Article received on September 1, 2010. Approved on October 1, 2010.