INTRODUCTIONThe search for adjuvant techniques to surgery is incessant, and the evolution of such techniques is no less important than that of the surgical procedure itself. The main goal of current research is the development of materials capable of enhancing the healing process and regulating inflammation.
One of the materials known to perform these functions is fibrin glue. While the use of fibrin glue in surgery is well documented, its availability is limited by the high cost of commercial material (1).
In this study, we demonstrate 3 methods of preparing fibrin glue from autologous plasma and compare the clot formation, toxicity, and resistance to traction of the resulting products.
OBJECTIVEThe preparation of fibrin tissue adhesive in the laboratory, assessment of the properties and characteristics of biological glue prepared by 3 different methods, and comparison of these traits among the methods.
MATERIALThe fibrinogen used to make the adhesive (component I of the glue) was prepared from blood obtained from 2 sources: blood bags from a blood bank (previously citrated to 3.8%) and randomly chosen patients (citrated to 10%). The additional materials used to manufacture component I were commercial sodium citrate (10%), a saturated solution of ammonium sulfate, and calcium chloride (40 mol/l). Component II of the glue, thrombin, was purchased commercially, as were aminocaproic acid and the distilled water used to resuspend the lyophilized powders of the products.
The effects of the products on healing were tested in New Zealand rabbits (3 groups of 5 rabbits; group 1: spin plasma, group 2: cryoprecipitation, and group 3: ammonium sulfate precipitation) and their strength and adhesiveness were investigated using fragments of human dura mater (5 tests with 2 fragments of dura mater.
METHODSFibrinogen isolation
The glue was prepared by extracting component I (fibrinogen) by 3 different methods. Component II was always the same commercial thrombin diluted in distilled water and mixed with aminocaproic acid (as an antifibrinolytic).
Method 1: Plasma: 36 ml of pre-citrated blood from the blood bank was divided among 4 tubes and centrifuged at 3000 rpm for 3 minutes. The plasma was removed by pipetting and mixed with 1 ml of calcium chloride solution.
Method 2: Plasma Cryoprecipitate: 350 ml of blood in a citrated bag frozen at -18°C was thawed in the refrigerator at 4°C and subjected to cold centrifugation at 5000 rpm for 5 minutes. Calcium chloride solution (1 ml) was added, and the precipitate at the bottom of the bag was used as component I of the glue.
Method 3: Plasma chemoprecipitate: a total of 36 ml of non-citrated blood was collected into 4 tubes containing 1 ml each of sodium citrate solution (10%) and centrifuged at 3000 rpm for 10 minutes. The plasma obtained was mixed with 1.3 ml of a saturated solution of ammonium sulfate in 4 siliconized tubes, which immediately precipitated part of the fibrinogen, and centrifuged at 3000 rpm for 3 minutes. About 1.5 ml of white precipitate was collected by siphoning and mixed with 1 ml of calcium chloride to make component I of the glue.
Tests:1. Clot formation: the time to form a highly adhesive clot and the duration of this clot were recorded.
2. The graft: rabbit skin grafts implanted using the various glues were evaluated for engraftment within 30 days, formation of granulation tissue, dislodgement/displacement of the graft, and scar formation.
3. Toxicity of the material: local and systemic manifestations of inflammation and/or allergic reaction were assessed.
4. Tensile strength: tensile test using dura mater fragments: 2 fragments of dura mater were attached with the fibrin glue, and the force required to detach them was measured using the amount of distilled water in the pipette as the weight (Figure 1).
RESULTSAll methods produced a large, adhesive clot that formed within 2 seconds and persisted unchanged for several hours. When the glues were used for skin grafting, group 1 rabbits did not engraft, as the grafts could be moved by gravity alone, while all grafts in groups 2 and 3 were clinically successful after 30 days. The rabbits' sclerae healed well with no evidence of toxicity in all groups. Method 3 produced the best tensile strength results in the dura mater test, requiring 39 g/cm² to separate the fragments vs. 23 g/cm² for the product of method 2 and 13 g/cm² for the product of method 1 (Figures 2 and 3).
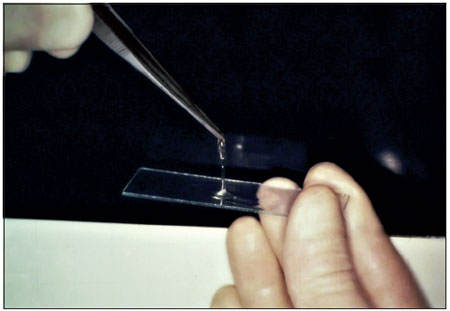
Figure 1. Fibrin glue.
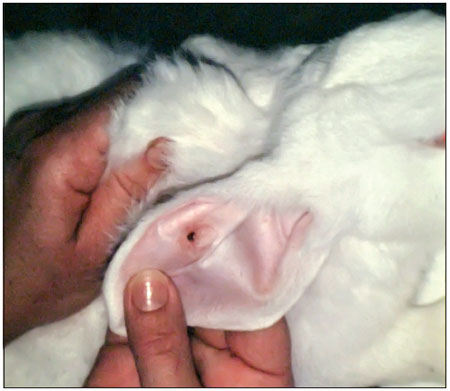
Figure 2. Handle with graft plasma.
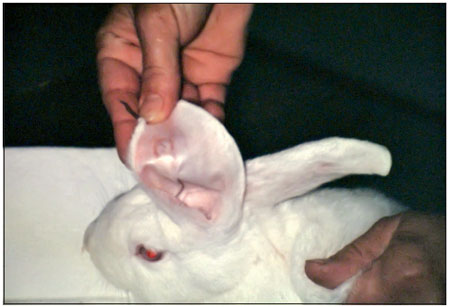
Figure 3. Handle with graft chemoprecipitate.
Despite the development of surgical techniques for hemostasis, the search for effective hemostatic agents continues. One such agent is fibrin glue.
Fibrin sealants reproduce the final stages of the enzymatic coagulation cascade, in which fibrinogen is converted to fibrin in the presence of thrombin, factor XIII, fibronectin, and ionic calcium (1,2).
Fibrin glue acts by inducing local hemostasis (modulation of the local inflammatory response) and/or by adhering apposed tissues (mechanical support) (2).
The use of fibrinogen adhesive remains limited by the high cost.
In this study, we demonstrated 3 methods for obtaining fibrinogen from blood for the production of fibrin glue and compared the products of these methods to determine which was best for surgical use.
Compared with other adhesives, such as cyanoacrylate and gelatin-formaldehyde-resorcinol, fibrin glue has the advantages of not causing tissue rejection reactions or stimulating the growth of fibroblasts; it is also completely biodegradable.
Precipitation of plasma proteins concentrates not only fibrinogen but also other desirable and undesirable components, such as XIII and plasminogen. Plasmin, a derivative of plasminogen, is the major proteolytic enzyme that acts to dissolve the clot. Due to this tendency of plasmin to destroy the fibrin clot, an antifibrinolytic agent such as aminocaproic acid should be added to glue component II in order to retard clot lysis and thus stabilize the tissues to allow scar formation.
Another advantage of fibrin adhesive is its low predisposition to infection. The growth of staphylococci in blood clots is 10 times higher than in fibrin clots and 100 times higher than in fibrin clots containing factor XIII (3).
Biological adhesives are used in several surgical procedures: closure of the dura, hemostasis, surgery of the middle and inner ears, septoplasty, facial nerve grafting, cerebrospinal fluid leak sealing CSF leaks, grafting of skin and mucous membranes, fracture stabilization, plastic surgery, and vascular, cardiac, thoracic, urologic, digestive, and dental microsurgery. The scope for its use is virtually unlimited, and the benefits are numerous (4,5,6,7,8).
Fibrinogen can be precipitated by various methods. Precipitation by the addition of a saturated solution of ammonium sulfate produces greater amounts of fibrinogen in a non-selective manner and brings down some additional proteins that are important in the clotting process (9).
In our study, autologous fibrin glues produced by 3 different methods formed clots identically well; however, skin grafting was equally successful when plasma cryoprecipitate or chemoprecipitate was used but unsuccessful when plasma precipitate was used.
The 3 methods were equally satisfactory with respect to local toxicity, as the rabbits' sclerae showed no signs of injury. Laboratory testing of the adhesion of dura mater fragments showed the worst results for glue produced from pure plasma, followed by that made from cryoprecipitate, while the best adhesion was obtained using chemoprecipitated plasma.
Although the adhesion of fibrin glue produced by chemoprecipitation was inferior to that of the commercial glue in the first 10 minutes, it has greater adhesiveness after 30 minutes (Siendentop, 1985) and would therefore be preferable in situations in which the fragments are not subjected to large shifts. Based on our results, fibrinogen prepared by chemoprecipitation from plasma is better for surgical use than that prepared by the other 2 methods.
Some surgeons now utilize the patient's own blood to prepare fibrin glue for the surgical site; this is both less expensive and more convenient for the patient than using commercial glue.
Furthermore, the patient's own blood poses none of the risks for contamination or infection associated with the use of blood from blood banks and also minimizes the risk for allergic reactions.
It is important to remember that the use of any biological or synthetic glue is no substitute for proper surgical technique.
CONCLUSIONFibrin glues produced by standard centrifugation, cryoprecipitation, and chemoprecipitation performed equally well at clot formation, and no method produced signs of toxicity in rabbit sclerae. However, the adhesiveness and perioperative graft healing were poor for the glue produced by centrifugation alone. Plasma chemoprecipitation produced the best results for tensile strength testing of adhesion between dural fragments. We therefore conclude that chemoprecipitation is the most effective method for preparing fibrin tissue adhesive.
REFERENCES1. Dohan S, Choukroun J, Dohan A, Donsimoni JM, Gabrieleff D, Fioretti F, Dohan D, Platelet Rich Fibrin (PRF). Un nouveau biomatériau de cicatrisation. Biotechnologies et fibrine, plaquettes et cytokines, aspects immunitaires, implications thérapeutiques. 1re partie: biotechnologies et fibrine. Implantodontie. 2004;13:87-97.
2. Reiss RF, Oz MC. Autologous Fibrin Glue: Production and Clinical Use. Transfusion Medicine Reviews, Vol X, No 2 (April), 1996, 86-92.
3. Almeida CIR, Nina LG, Mello RP. Cola biológica autógena. Rev Bras Otorrinolaringol. 1991;57:115-8.
4. Bento RF, Miniti. Comparison between fibrin tissue adhesive, epineural suture and natural union in intratemporal facial nerve of cats. Acta Oto-Laryngologica. 1989;465:1-36.
5. Jessen C, Sharma P. Use of fibrin glue in thoracic surgery. The Annals os Thoracic Surgery. 1985;39(6):521-4.
6. Tidrick RT, Seegers WH, Warner ED. Clinical experience with thrombin as na hemostatic agent. Surgery. 1943;14:191-6.
7. Bento RF, Salomone R, Tsuji RK, Housen M, Neto RB. O Uso de Cola de Fibrina Humana na Anastomose de Lesões Traumáticas Parciais do Nervo Facial. Arq. Int. Otorrinolaringol. 2008;12(2):214-219.
8. Sterkers O, Becherel P, Sterkers JM. Reparation du nerf facial par colle de fibrine exclusive 56 cas. Annals Otolaryngolo ic Chirurgical Cervicofacial. 1989;106:176-81.
9. Siedentop KH, Harris DM, Sanchez B. Autologous fibrin tissue. Laryngoscope. 1985, 9:1074-76.
1) Medical Resident in Otolaryngology, HC-UFPR (Hospital de Clínicas da Universidade Federal do Paraná).
2) MMSc. Physician Assistant, Department of Otolaryngology, Faculty of Medicine, UFPR; Associate Professor of Otolaryngology.
3) Otolaryngologist by ABORLCCF.
Institution: Hospital de Clínicas da Universidade Federal do Paraná. Curitiba / PR - Brazil. Mailing address: Juliana Benthien Cavichiolo - Rua General Carneiro, 181 - Alto da XV - Curitiba / PR - Brazil - Zip code: 80060-900 - E-mail: jucavs@hotmail.com
Article received on May 1, 2011. Article accepted on October 25, 2012.