INTRODUCTIONBalance is maintained through the interaction of 3 systems, the vestibular, visual, and proprioceptive systems. All of the afferent information from these systems is sent to the cerebellum, which harmonically coordinates, plans, and executes the automatic responses of the body that maintain static and dynamic balance. All of this information must be consistent and symmetrical because any conflicting data can generate dizziness or imbalance (1).
Dizziness is a feeling of disturbance of body balance that can be triggered by any change in the responses originating from the vestibular or central system. It can be classified as rotational or non-rotational. Rotational dizziness, or vertigo, originates fundamentally as an intensified response of the vestibular system (2).
Benign Paroxysmal Positional Vertigo (BPPV) is a fairly common vestibular disorder characterized by brief but intense episodes of rotational vertigo triggered by rapid movement of the head. The diagnosis of BPPV is primarily clinical and can be corroborated by the use of the Dix-Hallpike maneuver and otoneurological examination (3, 4, 5).
In patients with BPPV, the Dix-Hallpike maneuver is expected to produce nystagmus in response to rapid movements. This response occurs within a few seconds and exhibits a latency period, a limited duration, and fatigue (i.e., a diminished response) upon repetition of the causative maneuver. This positional nystagmus in patients with BPPV can be explained by the anatomy and physiology of the vestibular system. The endolymph contains freely moving particles, and the movement of detached statoconia (or calculations) that occurs in conjunction with movement of the head generates an abnormal acceleration of the endolymph, causing abnormal deflection of the cupula (3, 6).
Patients often describe the BPPV episodes as brief but intense periods of rotational vertigo triggered by rapid head movements. The triggering events most often reported are standing, lying down and turning over in bed, and looking up and down. Gait abnormalities, dizziness, and other types of disability are often reported between crises (7).
The quality of life of these patients is seriously impaired, as BPPV causes physical, functional (everyday activities), and emotional problems that are directly linked to psychological issues; these often progress to depression (8).
Digital vectoelectronystagmography (VENG) is an examination that can assess the integrity of the vestibular pathways. The description of the exam can be divided into 2 phases: first, oculomotor testing, which primarily assesses the different patterns of ocular movements, and then evaluation through direct stimulation of the semicircular canals, rotational chair testing (PRPD), and caloric testing (1, 9).
Patients with BPPV patients may present with findings related to each affected channel and its corresponding effects on the results of caloric testing. The findings range from normal reflexes to hyperreflexia or hyporeflexia contralateral or ipsilateral to the side affected by BPPV (4, 10, 11).
Another tool that can be used to evaluate the neural pathways of the vestibular system is Vestibular Evoked Myogenic Potential (VEMP) testing. This is a painless test that is rapid and easy to perform. The VEMP is a reflex triggered by a high-pitched sound in the ipsilateral ear; the response can be captured by surface electrodes. The latency, reliability, and distance between the peak (p13) and valley (n23) of the wave are used to assess whether the vestibular system is normal or altered. VEMP testing is complementary to VENG and provides valuable information that can help to solidify the diagnosis (12, 13).
VEMP testing is a relatively new examination that is an important supplement to digital vectoelectronystag¬mo¬graphy in otoneurologic diagnosis. The phonoaudiologist's role is to study the scope of this examination in order to enrich his or her clinical practice and to support and promote study of this area.
While BPPV is a fairly common peripheral otoneurological disorder, little is known of the VEMP findings in patients with this disease. Researching and describing these findings is very important both to increase the knowledge in the field and to open new therapeutic horizons. VENG quantitatively assesses the operation and interaction of the vestibulo-ocular reflex as well as the operation of the semicircular canals, as pairs or individually, while VEMP provides data regarding the vestibulospinal reflex (2,6) and the function of the otolithic organs, which are very important in the detection of linear acceleration. Together, these 2 methods complement the patient's reports and the clinical examination and provide the health professional with precious data on the mechanism of body balance beyond the limits of the vestibular labyrinth (14, 15). The object of this project was to relate the findings of VEMP testing and VENG in patients with BPPV.
METHODSThis was a retrospective, descriptive study performed by collecting and analyzing the results of vestibular exams and Evoked Myogenic Potential tests performed in the Otoneurology Division of Universidade Federal of São Paulo and Hospital Paulista.
The inclusion criteria used in this study were a diagnosis of BPPV by an otolaryngologist, age between 18 and 60 years, and the availability of the results of both VEMP testing and VENG.
The diagnosis of BPPV was made through examination by an otolaryngologist and monitoring, at the Otoneurology Clinic, of patients who complained of vertigo generated by the rapid movement of the head. The diagnosis considered the responses to tests such as the position and positioning maneuvers and the latency and fatigability of the nystagmus. This project included patients with posterior semicircular canal BPPV regardless of the physiological cause.
The examinations collected and analyzed were Digital Vectoelectronystagmography (VENG) and Evoked Myogenic Potential (VEMP) tests conducted between 2009 and 2011.
The VENG included normal vestibular exams, vestibular dysfunction with deficient unilateral or bilateral modification, and hyperreflexia.
The exclusion criteria included positive central vestibular exam findings (presence of signs and symptoms), age less than 18 years, exams performed before January 2009 or for which the results were incomplete (i.e., without a final diagnosis), absence of either VENG or VEMP results in the same patient, the presence of another vestibular disease, diseases of the external and middle ear that could interfere with the results of VEMP testing, use of medication that could affect the vestibular system and/or muscle tone, neuromuscular disorders, hearing impairment (unless deemed compatible with age, i.e., presbycusis), and history of vestibular rehabilitation. Patients who had not undergone only 1 of the tests (oculomotor phase of VENG: NEOA (spontaneous nystagmus with the eyes open), NEOF (spontaneous nystagmus with the eyes closed), NSE (semi-spontaneous nystagmus), RP (tracking movements), OPTO (optokinetic nystagmus), vestibular phase of VENG: PRPD or PC; VEMP testing: analysis of latencies or wave reproducibility) within each examination were included in the study. However, patients who failed to perform 2 or more of the tests listed above were excluded from the sample.
VENG was performed using registration equipment with VECWIN Software and an NGR - 05 air otocalorimeter with temperatures of 42 ºC and 18 ºC and the capacity to be chilled to 10 ºC if necessary, both from Neurograff Eletromedicina, Ltda. The tests that were included were: study of positional nystagmus using the Dix and Hallpike maneuver, calibration, spontaneous and semi-spontaneous nystagmus, saccades, pendular tracking, optokinetic nystagmus, rotational chair testing (PRPD), and caloric testing.
Are standards and recommendations are reported to all patients subject to search for greater uniformity of data: before the exam, the patient was instructed to suspend the use of medicine for the treatment of dizziness, tranquilizers, and muscle relaxants for 72 hours prior to the examination (vital medicine such as that for the treatment of heart disease or blood pressure control was not suspended) and to avoid food or other substances that stimulate the vestibular system (i.e., substances high in caffeine or stimulants, such as chocolate, cigarettes, soft drinks, alcoholic beverages, or tea) for 24 hours prior to the examination. For the day of the exam, the patients were also instructed to bring their most current audiometry results, to bring someone to accompany them, if possible, to fast for at least 3 hours before the exam, and not to wear makeup or contact lenses.
The reference values for the VENG testing used in this research were those of Costa and other authors (6, 9, 16, 17); these and the reference values for VEMP are shown in the frame(below) Chart 1.
The second part of the exam consisted of VEMP testing conducted with Navigator® equipment from Bio-logic Systems Corporation and AEP software version 6.2.0 in a soundproof environment. The sound stimuli were presented through insert earphones.
The skin where the electrodes were to be placed was cleaned with gauze and pulp scrub, and self-adhesive surface electrodes were placed on the skin with a hypoallergenic conductive gel; these measures ensured impedance equal to or less than 5 kilohms (K) for each electrode with a difference smaller than 2 K among them. The electrodes were positioned on the upper third of the sternocleidomastoid muscle ipsilateral to the side of sound stimulation (positive polarity), on the upper portion of the sternum (negative polarity), and on the upper third of the sternocleidomastoid muscle contralateral to the sound stimulation (ground electrode). During the presentation of the sound stimulus, the patient was asked to perform a 30o flexion of the head to the trunk to maintain the sternocleidomastoid muscles in a contracted state during registration, thus obtaining muscle activation between the sides. The response parameters were the absolute latencies of p13 and n23 in ms, and values greater than the mean plus 2 standard deviations (2SD) were interpreted as altered responses.
The tracings obtained from the first biphasic potential consisting of p13 and n23 corresponded to the reflex evoked by sound stimulation of the saccular macula.
The data were organized using a Microsoft Excel 2010 spreadsheet. SPSS (Statistical Product and Service Solution) version 16.0 and Minitab 15 were used for statistical analysis. The data were collected at the institution using spreadsheets. After the data were examined, statistical analysis was performed to ensure the validity of the results. The analysis of variance (ANOVA), Confidence Interval for Mean Calculator, and Equality of Two Proportions tests were performed. The P-value for each comparison was also determined and used with a significance level of 0.05 (5%). All confidence intervals used throughout the study represent 95% statistical confidence.
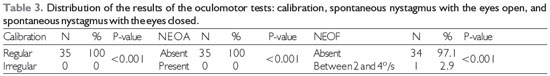
Legend: NEOA: spontaneous nystagmus with the eyes open; NEOF: spontaneous nystagmus with the eyes closed
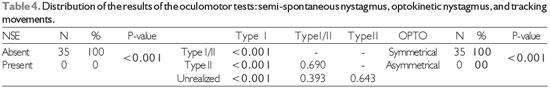
Legend: NSE: semi-spontaneous nystagmus; Type I, I/II, and II: tracking movements; OPTO: optokinetic nystagmus

Legend: PRPD*: decreasing pendular rotatory test.
Application of the inclusion and exclusion criteria yielded 35 patients, 26 women and 9 men. The results of 34 examinations were better for women than for men, in agreement with the findings of previous studies (11, 16 -20).The average age of the patients was 53 years, with a range of 51 to 60 years. The age distribution was quite similar to those in several previous studies of patients with BPPV, in which increasing age was cited as a predisposing factor for BPPV; one of the most plausible explanations for this is that age-related hormonal changes, especially in women in this age group, cause a massive loss of calcium, making the statocones less dense and more mobile (11,16,21) (Table 1).
Audiological evaluation showed a statistically significant relationship between normal unilateral hearing and contralateral sensorineural loss (PASNC), as shown in Table 2. This relationship (normal hearing + PASNC) is not frequently described in studies of patients with BPPV. Most research reports no changes in hearing in patients with BPPV; however, this finding may be suggestive owing to the association of BPPV with Ménière's disease, which produces frequent changes in hearing thresholds due to endolymphatic hidropisia15.
The data for the qualitative variables of VENG were analyzed by Equality of Two Proportions, with the following results Table 3.
Although the proportions of normal and abnormal findings were significantly different (p < 0.001), nothing was found that relates to the results of the oculomotor tests in patients with BPPV. No irregular calibration or NEOA should be expected, as their presence may suggest central vestibular disease (1).
Table 4 shows a pattern similar to that of Table 3: there was no NSE or asymmetry in the optokinetic nystagmus, both of which responses are considered pathognomonic for central vestibular alteration and therefore incompatible with the inclusion criteria of this study1. Analysis of the pendular tracking results showed a significantly (p > 0.001) greater proportion of Type I relative to Type I/II or Type II. For all tests, statistically significant differences were found between the proportions of normal and abnormal findings. The percentage findings are shown in detail below in Graph 1.
It is noteworthy the present study attempted to classify the RP according to its morphology (type I, I/II, II, or III), whereas previous studies have used quantitative variables, i.e., gain values; however, all studies obtained the same result: RP type I (9, 20, 22). Yet another study reported that age influenced the velocity of the slow phase of nystagmus (VACL) of the PR, thereby decreasing the gain values.
The OPTO results also exhibited statistical significance, as the result was symmetrical in all cases. This result, like those of the other tests, may indicate the absence of pathognomonic signs of central vestibular disorders; it was also similar to the findings of previous studies (1, 9, 20, 22).
Although most studies using VENG in patients with BPPV have prioritized only the vestibular testing (PRPD and caloric testing) (24-26), those in which the oculomotor tests were also examined showed results quite similar to those of the present study, with findings within the normal reference ranges in most cases.
The PRPD was performed in 34 patients, of whom 91.4% (N = 32), a statistically significant (p < 0.001) preponderance, showed symmetry between both the anterior and superior side channels, as shown in Table 5. This finding is also similar to those of previous studies (22). However, another study found no statistical significance in this regard (23) (Table 5).
Caloric testing showed normal reflexes in most (n = 20; 60%) cases, similar to the findings of some previous studies (4, 25). However, most of the studies converge on normoreflexia as the overall result of the test. This result can be explained by the fact that BPPV is a mechanical condition caused by displacement of the otoconia, which directly influences the operation of the lateral semicircular canals (the region evaluated by caloric testing) (23). In this study, normoreflexia was more prevalent than hyperreflexia in the left ear (LE) or hyperreflexia in the cold in both ears (Table 6 e 7).
Analysis of the etiology of BPPV showed statistical significance. Although the idiopathic form was the most prevalent, it was the etiology for which there was no statistical significance (see Table 8). This is in contrast to the findings of other studies that reported associations of idiopathic hidropisia (6, 13), trauma, and impact (16) with otological problems.
Examination of the side of onset showed that the left ear was more often affected than the right, while right onset was more prevalent in turn than bilateral onset. Although this difference was not statistically significant and there is no plausible pathophysiological explanation, this result was similar to those of other studies, with a recurrence rate in this study of approximately 69% (27-29,30).
The physiological cause of BPPV was more often ductolithiasis than cupulolithiasis. The percentages of these findings were very similar to those in several previous studies16.The rarity with which cupulolithiasis is associated with BPPV can be explained by the firm adhesion of the statocones to the cupulae, whereas in ductolithiasis these same particles float freely through the endolymph of the semicircular canals, facilitating the treatment (i.e., decreasing the number of repositioning maneuvers required) (19) (Table 9).
Analysis of the semicircular canal most often involved showed a massive and statistically significant (p < 0.001) predominance of the posterior canal (Table 10). Some studies with similar findings explain this result in terms of the anatomical position of this canal close to the saccular macula, where the otoconia originate (4, 10, 11, 14, 15, 16, 25).
BPPV recurred in a majority of patients (n = 20, 57.1%), as shown in Table 11. These results are very similar to those of more recent studies (6, 12, 13). The present study found a statistically significant association between recurrence and persistence, suggesting that if a patient experiences several episodes of BPPV, it is likely that the condition will become refractory to the positioning maneuver. Some authors propose that age influences recurrence, as aging usually leads to a decrease in neural activity combined with a decrease in the effectiveness of central inhibitory control, which implies more excessive reactions, as well as to progressive loss of calcium from the body, leading to less-dense statoconia requiring a greater number of maneuvers (21) (Table 11).
The VEMP waveforms were similar in all patients, with normal morphology but abnormalities in some of the quantitative parameters. Although the values for the right ear (Lp13od and Ln23od) were below the reference range adopted for this research6, the corresponding values between the peaks and the asymmetry index (AI) were within normal limits12, 13. Other studies have described such findings as prolonged latency and reduced amplitude in patients with BPPV13; these were generally associated with concomitant impairments12, 28. Another factor that influenced the research on this topic is the positive correlation between inter-peak amplitude and age30; in other words, as the individual with BPPV ages, the latency period of the reflex increases. However, the results of all studies agree that an altered VEMP is expected in patients with BPPV even when the results of caloric testing are normal12,13,27-29, and the low inter-peak latency in the patients' right ears in the present study corroborates this consensus.
The effect of the positioning maneuver was almost statistically significant and would likely have been significant in a study with a larger sample size; in other words, it is safe to say that the effects of the positioning maneuver on the VEMP results for the left ear tend to reflect the morphology and latencies of normal waves (Table 12). However, analysis of the VEMP results with respect to the audiological profile yielded no relevant statistically significant findings, as shown in Table 13.
Analysis of the caloric testing results with respect to the VEMP findings demonstrated that normoreflexia is associated with a normal inter-peak amplitude in the left ear. Studies have reported that age is a confounding factor, as the response and the chance of hyperreflexia both increase with increasing age (23), but normoreflexia was present in the absolute majority of cases in this sample despite the changes in the VEMP findings (12) described in Table 14. In other disorders, such as kinetosis, bithermal stimulation is expected to produce bilateral hyperreflexia. However, there are few publications in the field of otoneurology on the relationships among BPPV, VENG, and VEMP.
This study may help to clarify the relationship between VENG and VEMP results in patients with BPPV. The hearing threshold results indicate that patients with Benign Paroxysmal Positional Vertigo tend to have normal hearing and mild sensorineural hearing loss simultaneously; however, this phenomenon does not significantly affect the results of Vestibular Evoked Myogenic Potential testing.
All of the results of oculomotor testing were normal, and this finding was statistically significant. Furthermore, vestibular testing showed normal results and normal function of all of the semicircular canals. Caloric testing tended to show an influence of the inter-peak p13n23 in the left ear.
Based on this research, one would expect patients with BPPV to have normal results for both the oculomotor and vestibular components of VENG despite the correlation between mild contralateral and unilateral sensorineural hearing loss. However, at least 1 abnormal VEMP finding (increased latency in cases in which the positioning maneuver produces positional nystagmus) is expected in these cases.
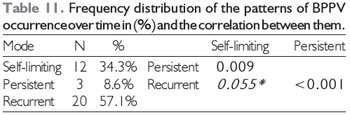
(*) trend towards significance.
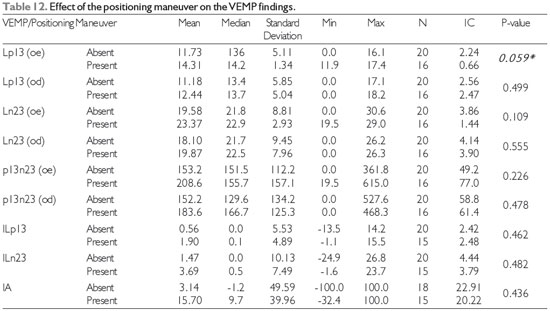
Legend: Min: minimum; Max: maximum; IC: confidence interval; VEMP: Vestibular Evoked Myogenic Potential
OD: right ear; OE: left ear
Lp13: Latency of the first positive deflection of the vestibular evoked myogenic potentials
Ln23: Latency of the first negative deflection of the vestibular evoked myogenic potentials
p13n23: Amplitude between the first positive deflection and the first negative deflection of the vestibular evoked myogenic potentials
IA: Index of the asymmetry amplitudes
ILp13: Index of the first positive deflection of the vestibular evoked myogenic potentials
ILn23: Index of the first negative deflection of the vestibular evoked myogenic potentials
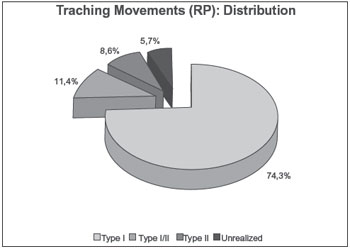
Graph 1. Frequency distribution of the tracking test results in (%).
The results of this research indicate that patients with BPPV are predominantly female, with an average age of 52.7 years, and have ductolithiasis as the physiological cause.
We can conclude that although there are average differences between the values of some Digital Vectoelectronystagmography variables with respect to the results of Vestibular Evoked Myogenic Potential testing, these effects are not significant. Therefore, we conclude that there were no associations between the results of audiologic evaluation, symmetry, positioning maneuver, or caloric testing and the quantitative results of Vestibular Evoked Myogenic Potential testing.
REFERENCES1. Ganança MM, Vieira RM, Caovilla HH. Princípios de otoneurologia. Vol. 1. São Paulo: Atheneu; 1998. Capther, Equilibriometria.
2. Ganança MM, Caovilla HH. Desequilíbrio e reequilíbrio. In: Ganança MM, editor. Vertigem tem cura?: o que aprendemos nestes últimos 30 anos. São Paulo: Lemos Editorial; 1998. p. 13-9.
3. Pereira CB, Scaff M. Vertigem de posicionamento paroxística benigna. Arq. Neuro-Psiquiatr. [Internet]. 2001 June [cited 2011 Apr 28];59(2B):466-70. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-282X2001000300031&lng=en. doi: 10.1590/S0004-282X2001000300031.
4. Manso A, Ganança CF, Ganança FF, Ganança MM, Caovilla HH. Achados à prova calórica e canal semicircular acometido na vertigem posicional paroxística benigna. Rev. soc. bras. fonoaudiol. [Internet]. 2009 [cited 2011 May 01];14(1):91-7. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-80342009000100015&lng=pt. doi: 10.1590/S1516-80342009000100015.
5. Costa VSP, Marchiori LL, Melo JJ, Guedes RFRP, Amâncio MK, Fontana AD, et al. Avaliação da manobra de reposicionamento de Epley em indivíduos com vertigem posicional paroxística benigna. Rev. CEFAC [Internet]. 2010 Oct [cited 2011 May 06];12(5):727-32. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-18462010000500002&lng=pt. Epub 23-Abr-2010. doi: 10.1590/S1516-18462010005000023.
6. Dorigueto RS. Estudo do potencial evocado miogênico vestibular na vertigem posicional paroxística benigna [dissertation]. São Paulo: Universidade Federal de São Paulo, Curso de Medicina, Departamento de Otorrinolaringologia e Cirurgia de Cabeça e Pescoço, 2010.
7. Kasse CA, Santana GG, Scharlach RC, Gazzola JM, Branco FCB, Doná F. Resultados do Balance Rehabilitation Unit na Vertigem Posicional Paroxística Benigna. Braz. j. otorhinolaryngol. (Impr.) [Internet]. 2010 Oct [cited 2011 May 21];76(5): 623-9. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-86942010000500015&lng=pt.
8. Pereira AB, Santos JN, Volpe FM. Efeito da manobra de Epley na qualidade de vida dos pacientes com vertigem posicional paroxística benigna. Braz. j. otorhinolaryngol. (Impr.) [Internet]. 2010 Dec [cited 2011 May 21];76(6):704-8. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-86942010000600006&lng=pt.
9. Costa KCF, Silva SMR, Ganança CF. Estudo das provas oculomotoras e vestibulares por meio da vectoeletronistagmografia digital. Disturb.Comun [internet] 2005 Dec [cited 2011 May 25];17(3):315-22 Available from: http://www.pucsp.br/revistadisturbios/artigos/Artigo_420.pdf.
10. Chung KW, Park KN, Ko MH, Jeon HK, Choi JY, Cho YS et al. Incidence of horizontal canal benign paroxysmal positional vertigo as a function of the duration of symptoms. Otol Neurotol. [Internet] 2009 Feb [cited 2011 May 20];30(2):202-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19180679?dopt=Abstract
11. Moon SY, Kim JS, Kim BK, Kim JI, Lee H, Son SI et al. Clinical characteristics of benign paroxysmal positional vertigo in Korea: a multicenter study. Korean Med Sci. [Internet] 2006 Jun [cited 2011 May 20];21(3):539-43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16778402
12. Murofushi T, Shimizu K, Takegoshi H, Cheng PW. Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch Otolaryngol Head Neck Surg. 2001 Sep;127(9):1069-72.
13. Akkuzu G, Akkuzu B, Ozluoglu LN. Vestibular evoked myogenic potentials in benign paroxysmal positional vertigo and Meniere's disease. Eur Arch Otorhinolaryngol. 2006 Jun;263(6):510-7.
14. Ganança FF. Tratamento da Vertigem e de outras tonturas. São Paulo: Lemos; 2002. p. 28-34.
15. Ganança MM, et al. Lidando com a Vertigem Posicional Paroxística Benigna. Acta ORL [Internet]. 2005 jan [cited 2011 July 31];23(1). Available from: http://www.actaorl.com.br/detalhe_artigo.asp?id=57
16. Caldas MA, Ganança CF Ganança FF, Ganança MM, Caovilla HH. Vertigem posicional paroxística benigna: caracterização clínica. Braz. j. otorhinolaryngol. (Impr.) [Internet]. 2009 Ago [cited 2011 Aug 06];75(4):502-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-86942009000400006&lng=pt
17. Lee NH, Ban JH, Lee KC, Kim SM. Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg. [Internet] 2010 Sep[cited 2011 Aug 05];143(3):413-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20723780
18. Atas A, Aksoy A, Turan E. VENG Abnormalties. In: BPPV. [Internet]. 10th Congress of the German Society of Audiology; 2007 Jun 06-09. Heidelberg; Germany; 2007. [Cited 2011 Oct 01]. Available from: http://www.uzh.ch/orl/dga2007/program/scientificprogram/Atas__A._et_al.pdf
19. Tuma VC, Ganança C,Ganança FF, Ganança MM, Caovilla HH. Avaliação oculomotora em pacientes com disfunção vestibular periférica. Rev. Bras. Otorrinolaringol. [Internet]. 2006 Jun [cited 2011 Apr 28];72(3):407-13. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-72992006000300019&lng=pt. DOI: 10.1590/S0034-72992006000300019.
20. Lourenço EA, Lopes KC, Pontes Jr. Á, Oliveira MH, Umemura A, Vargas AL. Distribuição dos achados otoneurológicos em pacientes com disfunção vestíbulo-coclear. Rev. Bras. Otorrinolaringol. [Internet]. 2005 Jun [cited 2011 May 22];71(3):288-96. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-72992005000300005&lng=pt.
21. Korres SG, Balatsouras DG, Ferekidis E. Electronystagmographic findings inbenign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. [Internet]. 2004 Apr [cited 2011 Oct 14];113(4):313-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15112976
22. Maia Roberto A, Diniz FL, Carlesse A. Manobras de reposicionamento no tratamento da vertigem paroxística posicional benigna. Rev. Bras. Otorrinolaringol. [Internet]. 2001 Set [cited 2011 Oct 10];67(5):612-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-72992001000500003&lng=pt.
23. Furman JM, Cass SP. Laboratory evaluation: Part Il Vectronystagmography and rotational testing. In: Baloh RW, Halmagyi GM, editors. Disorders of the Vestibular System. New York: Oxford University Press; 1996. p. 191-210.
24. Felipe L, Santos MAR, Gonçalves DU. Potencial evocado miogênico vestibular (Vemp): avaliação das respostas em indivíduos normais. Pró-Fono R. Atual. Cient. [Internet]. 2008 Dec [cited 2011 Oct 17];20(4):249-54. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-56872008000400008&lng=en.
25. Korres S, Gkoritsa E, Giannakakou-Razelou D, Yiotakis I, Riga M, Nikolpoulos TP. Vestibular evoked myogenic potentials in patients with BPPV. Med Sci Monit. 2011 Jan;17(1):CR42-47.
1) Resident in Multiprofessional Hospital Care (concentration area: Adult and Elderly), University Federal of São Paulo UNIFESP. Speech Therapist.
2) Doctorate in Science from the Federal University of São Paulo. Member of the Brazilian Society of Otolaryngology.
3) PhD in Human Communication Disorders, Federal University of São Paulo, UNIFESP, Brazil. Visiting Professor of Otoneurology in the Undergraduate and Post Graduate Speech Therapy Program, UNIFESP.
Institution: Resident in Speech Lenguage. Escola Paulista de Medicina - Multidisciplinary Residency in Hospital Care - Department of Otorhinolaryngology. Teresina / PI - Brazil. Mailing address: Fga Res. Marta Lira - Antônio Bona St, 539 - São Cristóvão - Teresina / PI - Brazil - Zip code: 64056-200. Otoneurotology Clinic
Article received on August 19th , 2012. Article accepted on December 25th , 2012.