INTRODUCTIONPlasma cells are mature B lymphocytes typically found in high or low quantity in various tissues and organs and in inflammatory foci in general. Their main function is the production of immunoglobulins or antibodies. Plasmacytic tumors refer to a group of lymphoproliferative disorders characterized by monoclonal expansion of plasma cells that produce a single immunoglobulin molecule (1-4).
Multiple myeloma is the most common type of plasmacytic tumor. However, it represents only 1% of all cancers and has an incidence of 3 new cases per 100,000 per year (4).
The classic triad in the diagnosis of multiple myeloma is the detection of M protein in the serum or urine (Bence-Jones proteins), greater than 10% plasmacytosis in the bone marrow, and the presence of osteolytic lesions by radiography.
Otolaryngologic manifestations of myeloma dyscrasias are rare, with solitary bone plasmacytoma and extramedullary plasmacytoma deserving emphasis.
Several publications indicate that solitary bone plasmacytoma is an initial manifestation of multiple myeloma, which may result in disseminated disease (5,6). It is represented by a solitary osteolytic lesion without systemic manifestations of multiple myeloma, and therefore has a favorable prognosis after treatment. It mainly affects the long bones, causing marked proteinuria and bone pain. These lesions are rare in the head and neck, but when they occur, they radiographically present as large multicystic areas of bone rarefaction, which is unlike the small and well-defined destructive bone lesions of multiple myeloma. The spread of solitary bone plasmacytoma for myeloma usually occurs 3-5 years after the primary diagnosis.
Extramedullary plasmacytoma is a plasma cell tumor that forms in soft tissues such as the lymph nodes, skin, and mucosa; thus, by definition, this tumor has no origin in the myeloid tissue of the bone. The tumor mass is not histologically distinguishable from myeloma. It cannot be stated with certainty that the localized manifestation of the disease is a precursor of multiple myeloma, which seems to occur with the solitary bone subtype (3,4). Extramedullary plasmacytoma corresponds to less than 10% of all plasmacytic tumors (1,2), representing less than 1% of all head and neck tumors (4) and less than 0.5% of tumors of the aerodigestive tract (3). It is present as multiple lesions in 10 to 20% of cases, and the tumor may compromise the pleura, mediastinum, spermatic cord, ovary, intestine, kidney, pancreas, lung, and skin in addition to the submucosa of the airways (7).
This extramedullary tumor was first described in 1905 by Schridde (8). The estimated global incidence of the disease is 1 case per 500,000 people (1,2).
It is important for the otolaryngologist to acquire knowledge of this disease since 80 to 90% of the extramedullary plasmacytoma cases are found in the head and neck (4,9); these tumors mainly occur in the respiratory tract, especially the submucosa of the nasal cavity, paranasal sinuses, nasopharynx, oropharynx, and larynx. This disease occurs in people above 40 years of age (over 95% of cases) (4), typically between the sixth and seventh decades of life, affects 3 to 4 times more men than women, especially in Caucasians, and exhibits a slowly evolving nature.
The clinical symptoms are more related to the specific location of the injury than to the nature of the tumor. In a series of 20 patients with extramedullary plasmacytoma of the head and neck, Kapadia and colleagues observed the following major symptoms: tumor or local edema in 80%, nasal obstruction in 35%, epistaxis in 35%, localized pain in 20%, proptosis in 15%, rhinorrhea in 10%, regional lymphadenopathy in 10%, and paralysis of the VI cranial nerve in 5% of cases (4,10).
Plasmacytoma appears as a circumscribed tumor and it can be confined to the submucosa or present an ulcerated surface. The tumor has a firm consistency, which can be sessile or pedunculated, the color ranges from gray-red to gray, and easy bleeding is often observed under manipulation.
When these tumors occupy the nasal cavities, a differential diagnosis should be conducted in order to rule out other bleeding tumors, especially squamous cell carcinomas. When determining the presence of a clone of plasma cells in the biopsy, an effective analysis must be performed to confirm the existing plasma cell disorder.
Herein, we describe a case of extramedullary plasmacytoma of the nasal cavity and paranasal sinuses that appeared with nasal bleeding. This case is considered cured after 6 years of multidisciplinary treatment. An analysis of the findings is also presented in relation to a literary review.
CASE REPORTEMPM, 51-year-old woman sought the otorhinolaryngology service of a tertiary University Hospital in September 2004 with progressive nasal obstruction that had lasted for the last 6 months. The obstruction was worse on the right and was accompanied by self-limited epistaxis. Her background indicated a history of smoking (30 years packet) and social drinking.
Upon ears, nose, and throat (ENT) examination, the patient felt pain on palpation of the right maxilla and a hardened tumor was observed on the right of the nasal pyramid with dimensions of 2.0 x 1.0 cm. The tumor was fixed, painful, exhibited an infiltrative aspect, and had erased the naso-genial furrow on the right. Anterior rhinoscopy revealed a pale-reddish, broad tumor that occupied about 96% of the right nasal cavity and apparently adhered to the side wall. The tumor had a smooth surface; the blood vessels were visible owing to their transparency and bleeding, and slight pain was experienced on tactile stimulation. In the left nostril, the patient had a blocked septum deviation to the left. In the mouth, a tumor approximately 2.0 cm in size with a rough surface and a pinkish color was observed on the upper gingival-labial groove. This tumor was grooved in the center by the upper denture and was topographically located from the right incisor to the first right molar. The tumor shared a markedly clear border with the buccal mucosa, with no signs of recent bleeding.
Computed tomography (CT) of the sinuses revealed expansive formation with soft tissue attenuation occupying the nasal passages to the choanae, especially on the right (Figure 1A and B). The expansion had caused thinning of the bone structures, with areas of discontinuity in the medial and inferior wall of the right maxillary sinus and hard palate, and the nasal septum also tapered off to the left.
Incisional biopsy of a lesion of the right nasal cavity was performed under local anesthesia. Hematoxylin and Eosin staining showed a nasal mucosa cut presenting neoplastic growth in the chorion consisting of a mantle of plasma cells in monotonous arrangement. Immunohistochemical assays demonstrated kappa chain clonality, with plasmacytoma features.
The patient underwent a thorough hematologic evaluation, including bone marrow, bone imaging, protein electrophoresis, and Bence-Jones proteinuria. This evaluation excluded multiple myeloma and demonstrated that her condition was extramedullary plasmacytoma. Radiotherapy was initially indicated, reaching a total dose of 4860 cGy into 3 facial chambers during 27 sessions. The sessions were halted ahead of schedule due to episodes of recurrent epistaxis, with partial improvement of nasal symptoms and a decrease in the size of the plasmacytoma. Approximately 12 months after the diagnosis, a surgical approach was decided by exposure via degloving and transantral access (Figure 2A and B). The tumor occupied the maxillary sinus with destruction of its side wall and invaded the right nasal cavity, completely displacing the septum to the opposite side. Upon removal of the tumor, bone erosion of the hard palate was observed, which exposed an oroantral and oronasal fistula in the right gingival-labial region. We attempted to close the fistula at the same time as surgical excision. However, persistence of the oro-nasal fistula was observed as of the 15th postoperative day.
During the subsequent 6 years of monitoring, the patient has undergone 3 procedures for closing the fistula, without success. The first procedure utilized a titanium mesh and screw, but the patient developed mucosal retraction and exposure of the metallic material after approximately 30 days. In the second procedure, the material fixed in the first procedure was excised, with an attempt to close the fistula by using a buccal mucosa flap; however, this procedure was not successful. In the last procedure performed, a flap rotation of the soft palate was chosen, after which the patient developed a small punctiform fistula.
The patient has remained under multidisciplinary, ENT, and hematology monitoring for a total of 6 years and has not exhibited evidence of systemic disease up to this point.
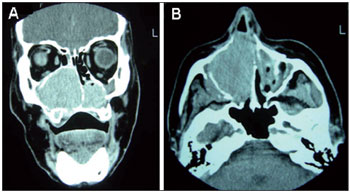
Figure 1. A and B. CT scan of the sinuses in the coronal and axial planes. The right nasal cavity and right maxillary sinus are occupied with material with soft tissue density, and signs of erosion of the maxillary floor are evident.
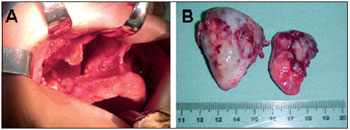
Figure 2. A and B. Exposure via degloving and transantral access that allowed excision of the tumor occupying the nasal cavity and right maxillary sinus. The macroscopic appearance of the extramedullary plasmacytoma that was surgically removed via 2 fragments of approximately 4 x 2 x 1 cm and 5 x 3 x 3 cm.
The diagnosis of extramedullary plasmacytoma depends initially on clinical suspicion. Deep biopsies should be performed because the tumor is submucosal and the mucosal lining can become thicker due to an inflammatory reaction. Aspiration with fine needles can hamper the analysis of the material because a common feature of malignant nasal tumors is a thickened tumor surface or secondary contamination of the tumor surface.
Because extramedullary plasmacytoma is rare, the first diagnosis of these tumors is usually a malignant tumor hypothesized to be squamous cell carcinoma since the clinical presentation of these entities is similar. In addition, histopathological analysis cannot distinguish a multiple myeloma from an extramedullary plasmacytoma, and further evaluation should be performed to rule out systemic disease.
A study using immunohistochemical methods confirmed the plasmatic nature of the cells with cell markers, e.g., CD 138, which indicates the necessity of performing an evaluation for a differential diagnosis from other cancers (melanoma, undifferentiated carcinoma, pituitary adenoma, and others) (6).
Immunohistochemistry of extramedullary plasmacytoma also differentiates the inflammatory processes, which are rich in the plasma cells in this region. The search for light chains allows the determination of whether the process is monoclonal, i.e., KAPPA or LAMBDA chain.
A negative bone marrow biopsy, the absence of bone lysis, and normal electrophoresis of blood immunoglobulins allows the possibility of a multiple myeloma to be excluded.
Thus, a diagnosis can be made by observing the following features (1,4):
1. Histological evidence of injury
2. Bone marrow aspiration showing less than 10% atypical plasma cells
3. Absence of clinical and radiological evidence of skeletal lesions
4. Unchanged levels of serum proteins or electrophoresis of serum/urinary proteins
5. Absence of anemia (80-85% patients with multiple myeloma have normochromic normocytic anemia)
Regarding the natural history of the disease, Batsakis (5) defined 5 possible stages that extramedullary plasmacytoma can present:
1. Localized disease; solitary; controlled by surgery, radiotherapy, or both; without recurrence; or disseminated.
2. Disease with local recurrence controlled by additional therapy
3. Aggressive disease, persistent or recurrent; death by uncontrollable local extensions.
4. Local disease with regional lymph node "metastasis" without evidence of distant spread.
5. Local disease, recurrent or followed by dissemination and development of another neoplasm of plasma cells and/or multiple myeloma.
Following this classification, the patient described after 6 years of monitoring is in stage 1.
Wiltshaw (9) stated in his study that 40% of extramedullary plasmacytomas spread beyond the primary site and/or undergo lymphatic drainage. Of these, 62% of patients had deposits in soft tissues and visceral organs, and 81% developed bone lesions. An extramedullary plasmacytoma at this stage is called Disseminated Extramedullary Plasmacytoma, and its prognosis is undoubtedly better than that of multiple myeloma. However, the possibility for conversion of extramedullary plasmacytoma to multiple myeloma exists. Its incidence rate varies from 15 to 20% and its prognosis is poor (10).
Regarding the treatment of extramedullary plasmacytoma in the head and neck, the choice of radiotherapy or surgery alone is controversial in the published data. Some authors advocate the use of radiotherapy as a sole treatment due to the good response in most cases. Chemotherapy is indicated only in cases of disseminated disease (11,12). In 1993, Wax (13) recommended surgery in cases of localized lesions that could be removed with minimal morbidity.
Regarding nodal dissemination (which occurs rarely) and local relapse, controversy persists because although metastases respond with the same sensitivity to radiotherapy, isolated surgery may also be indicated. In 1988, Abemayor recommended the exclusive use of radiotherapy and suggested reserving surgery only to remove residual disease (14).
Although it is known that extramedullary plasmacytoma is a radiosensitive tumor, no consensus exists in the literature regarding the optimal dose for therapy. Various protocols have been described, with a total dose ranging from 3,000 to 8,000 rads over treatment periods of 3 and 6 weeks (4).
Furthermore, the slow regression of the tumors treated with radiotherapy has been reported, with no consensus on the maximum time for the radiation effect. Radiotherapy and surgery can be combined, and this approach is used mainly when a tumor remains. A smaller tumor remained in the reported case, facilitating its resection after receiving radiotherapy.
Regarding the results of the possible treatments, in 1976, Wilthaw (9) obtained a local recurrence rate of 21% for radiotherapy alone, 20% for surgery alone, and 46% for combination therapy, demonstrating that the requirement for combination therapy reflected more severe cases. Factors associated with a worse prognosis in the literature are as follows:
- presence of bone destruction;
- large primary tumor;
- recurrence; and,
- tumors located in the sphenoid sinus, maxillary sinus, or larynx.
All cases justify long-term monitoring because extramedullary plasmacytoma can recur as disseminated multiple myeloma and the evolution of this tumor is unpredictable (3). Shah et al. (15) recommend that the patient be monitored 28 and 36 months after primary treatment. The disease has a 65% expected 5-year survival rate (19) and median survival ranges from 4 to 10 years (4).
The patient in the study completed 72 months of follow-up without reporting complaints regarding tumor recurrence. The patient only required a prosthesis for feeding and to prevent nasal reflux after 3 failed procedures were performed to correct the fistula.
FINAL COMMENTSExtramedullary plasmacytoma is a rare, aggressive tumor that mainly affects the submucosa of the nasal cavity and paranasal sinuses. This tumor can remain in the area of the early lesion, advance to neighboring areas, or even spread. The otorhinolaryngologist must identify the lesion and refer the patient for hematologic monitoring; moreover, a multidisciplinary approach is required to differentiate between localized disease and blood dyscrasias with a poor prognosis, such as multiple myeloma. The prognosis of extramedullary plasmacytoma is much more favorable than that of multiple myeloma. Treatment with radiotherapy is effective because the tumor is radiosensitive, and surgery may occasionally be used to complement the treatment. Controlled clinical trials are needed to establish a definitive treatment of choice for the management of these patients. The patient should always be monitored for a long period of time.
REFERENCES1. Fernandes AM, Podovani JA, Maniglia JP. Plasmocitoma extramedular de nasofaringe: relato de um caso e revisão da literatura. Rev Bras Otorrinolaringol. 1998;64:296-8.
2. Sousa RMA, Costa EG, Takahashi GM, Butugan O, Miniti A. Mieloma múltiplo e manifestações raras dentro da otorrinolaringologia. Rev Bras Otorrinolaringol. 1993;59:284-8.
3. Granato L, Petitto JW, Prospero JD. Plasmocitoma extramedular do aparelho respiratório Apresentação do caso. Rev Bras Otorrinolaringol. 1977;43:214-23.
4. Kuppersmith RB. Extramedullary plasmocytoma of the head and neck [Internet]. [Cited 1996]. Available from: http://www.bcm.edu/oto/grand/32896.html
5. Batsakis JG. Plasma cell tumors of the head and neck. Ann Otol Rhinol Laryngol. 1992;92:311-3.
6. Creston JE. The otolaryngologic manifestations of multiple myeloma. Laryngoscope. 1978;88:1320-32.
7. Hidaka H, Ikeda K, Oshima T, Ohtani H, Suzuki H, Takasaka T. A case of extramedular plasmocytoma arising from the nasal septum. J Laryngol Otol. 2000;114(1):53-5.
8. Shridde H. Weitere Untersuchungen uber die Kornelunger der Plasmazellen. Centralbl Allg Pathol Anatol. 1905;16:433-5.
9. Wiltshaw E. The natural history of extramedullary plasmocitoma and its relation to solitary mieloma of bone and myelomatosis. Medicine. 1976;55:217-38.
10. Upall HS, Harrison P. Extramedular Plasmocitoma of the larynx presenting with upper airway obstructionin in a patient with longstanding IgD myeloma. J Laryngol Otol. 2001;115(9):745-6.
11. Kapadia SB, Desai U, Cheng VS. Extramedullary plasmacytoma of the head and neck. A clinicopathologic study of 20 cases. Medicine. 1982;61(5):317-29.
12. Paris J, Dessi P, Moulin G, Chrestian MA, Braccini F, Zanaret M. Plasmocytome solitaire extra-medullaire de la fosse nasale: a propos dun cas. Rev Laryngol Otol Rhinol. 1999;120:343-5.
13. Wax MK, Yun KJ, Omar RA. Extramedullary plasmocytomas of the head and neck. Otolaryngol Head Neck Surg. 1993;109:877-85.
14. Abemayor E, Canalis RF, Greenberg P, Wortham DG, Rowland JP, Sun NC. Plasma cell tumors of the head and neck. J Otolaryngol. 1988;17(7):376-81.
15. Shah C, Roy D, Naronha B. Extramedullary plasmacytoma of the submandibular gland. J Laryngol Otol. 2001;115(12):1023-5.
1) MSc in Otorhinolaryngology. Assistant Professor, Department of Otorhinolaryngology, Santa Casa de Misericórdia de São Paulo.
2) PhD in Otorhinolaryngology, Federal University of São Paulo. Associate Professor, Department of Otorhinolaryngology, Irmandade da Santa Casa de Misericórdia de São Paulo.
3) Fellow in Otolaryngology, Irmandade da Santa Casa de Misericórdia de São Paulo.
4) PhD. Associate Professor, Department of Pathology, Irmandade da Santa Casa de Misericórdia de São Paulo.
Institution: Irmandade da Santa Casa de Misericórdia de São Paulo. São Paulo/SP - Brazil. Mailing address: Marco Antonio dos Anjos Corvo - Al. Joaquim Eugênio de Lima, 1601, apto 141 - Jardim Paulista - São Paulo / SP - Brazil - Zip Code: 01403-003 - Telephone: (+55 11) 3283-4789 - E-mail: marcoantoniocorvo@hotmail.com
Article received on March 2, 2011. Article accepted on May 21, 2011.