INTRODUCTIONHamartomas of the head and neck generally, as well as the sinonasal tract specifically, are very rare. They often consist of mesenchyme and may rarely include epithelial elements. Respiratory epithelial adenomatoid hamartoma (REAH) and chondro-osseous respiratory epithelial adenomatoid hamartoma (COREAH) are examples of hamartomas comprising both epithelial and mesenchymal components. Although invariably benign, these lesions may grow to relatively large sizes and pose clinical concerns. We report herein what represents, to the best of our knowledge, the third case of COREAH of the nasal cavity, emphasizing its clinicopathologic features and the importance of recognizing and differentiating it from other, potentially more aggressive lesions of the nasopharynx.
CASE REPORTA healthy 38 year-old woman presented to our institution with a left nasal mass that was discovered on a computed tomography (CT) scan performed at an outside hospital. She had complained of mild left nasal obstruction of a few months' duration; however, no epistaxis, rhinorrhea, or hyposmia was reported. A punch biopsy obtained at the referring institution was interpreted as benign respiratory epithelium (slides not reviewed).
The CT scan was repeated and confirmed the presence of a 4.0x2.4x1.1-cm soft tissue mass with calcification located in the posterior aspect of the left nasal cavity. The mass abutted the lateral wall of the cavity, extending into the left sphenopalatine foramen and the left ethmoid air cells, and protruded into the nasopharynx posteriorly (Figure 1). No associated bone erosion was seen. Endoscopic examination revealed a lobulated, well-circumscribed, smooth, tan-white polypoid lesion occupying most of the left nasal cavity. The lesion was not attached to any structure inside the nasal cavity proper, but its posterior aspect was minimally attached to the lateral nasopharyngeal wall. Endoscopic surgery was performed, and the mass was removed completely.
Pathology
The submitted specimen measured 4.0x2.3x1.1 cm, had a tan, smooth outer surface, and was accompanied by multiple small irregular fragments of soft and bony tissue. The mass was hard and polypoid with a homogenous tan-white cut surface. Microscopic sections exhibited respiratory-type epithelium with underlying submucosal glands, hypocellular fibromyxoid stroma, and mature bone. The submucosal glands were irregular and exhibited focal mucinous metaplasia, but no significant thickening of the basement membrane was seen. The gland lumens were filled with mucoid material. Scattered chronic inflammatory cells were noted throughout the lesion. There was no evidence of atypia (Figure 2).
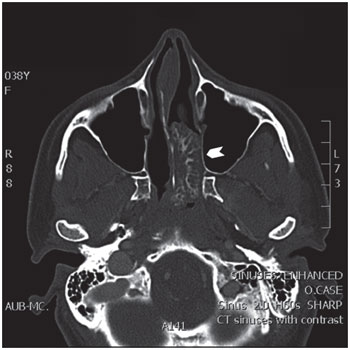
Figure 1. Axial computed tomography (CT) image of a left nasal chondro-osseous respiratory epithelial adenomatoid hamartoma (COREAH) showing a large (4x2.4x1.1 cm), iso-intense mass with focal enhancement (arrowhead) causing mild deviation of the nasal septum to the right with no erosion of the bone.
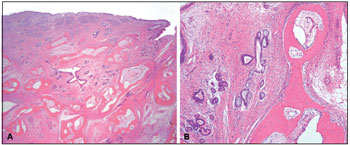
Figure 2. A. COREAH. Submucosal glandular proliferation originating from surface respiratory epithelium-lined mucosa, hypocellular fibromyxoid stroma, and mature bone. (Hematoxylin & eosin, 20x) B. COREAH. Mucinous metaplasia and mature bone trabeculae (osseous metaplasia). (Hematoxylin & eosin, 100x)
Hamartoma is defined as a mass-forming aberrant organization of specialized cellular components indigenous to a particular body site (1). It does not clearly represent a neoplastic or an inflammatory process or have the capacity for continuous unimpeded growth (2). Hamartomas have rarely been reported to arise in the sinonasal region. Until the early 1990s, the majority of the sinonasal hamartomas were reported to be mesenchymal, most frequently vascular, in origin (3). However, epithelial hamartomas, namely REAHs, have since been more frequently reported (2,4-6). REAH was originally described in 1995 by Wenig and Heffner, who included in their series 1 case containing mixed epithelial and mesenchymal elements with osseous metaplasia (2). In a later abstract presentation, the authors designated this chondro-osseous lesion as COREAH (4). A formal literature review revealed only 2 other reports of COREAH (5,6), making ours the third such case published.
Two hypotheses have been proposed for the etiology of REAH (and by extrapolation COREAH). The first is that REAH, similar to other hamartomatous lesions, is congenital in nature and results from an inborn developmental error. The second explanation is that given the association of such hamartomas with chronic sinusitis, they may represent the effect of the inflammatory process on inflammatory polyps (2). One study that investigated the molecular genetic changes in REAH revealed a higher loss of heterozygosity (LOH) at loci located on chromosome 9p and 18q than expected for a benign lesion (7). Interestingly, the 9p region is known to code for 2 structurally distant tumor suppressor proteins and is frequently affected in human neoplasms, while LOH at 18q has been shown to be common in recurrent or metastatic squamous cell carcinoma of the head and neck and may therefore play a role in tumor progression (7). Nevertheless, despite these reported molecular alterations, REAH and COREAH are completely benign lesions.
Clinically, REAH has been noted to arise in the posterior nasal cavity in the majority of cases (2). However, other sites, including the nasopharynx and the ethmoid, maxillary, and frontal sinuses, have been described (2,8). The presenting symptoms include nasal obstruction and stuffiness, chronic sinusitis, rhinorrhea, hyposmia, and headache (8).
The 2 COREAH lesions previously reported in the literature were also located in the nasal cavity. Grossly, they were fleshy to firm and polypoid, with white smooth outer surfaces and solid cut surfaces with occasional mucus filled cysts (5,6). Microscopically, they resembled REAH, with features including a predominantly glandular submucosal proliferation with round to oval glands varying from small to markedly dilated and lined by ciliated respiratory epithelium originating from the surface mucosa. These glands were separated by stromal tissue with no evidence of complex growth or cribriform architecture. Mucinous metaplasia with mucoid or amorphous material filling the glandular lumens was occasionally seen. Other histological features included changes typically seen in association with inflammatory sinonasal polyps, namely stromal edema, polypoid growth, increased numbers of seromucinous glands, vascular and fibroblastic proliferation, and mixed acute and chronic inflammatory cells (2,5,6). Dysplastic or neoplastic changes were not reported in REAH or COREAH.
Glandular hamartomas, inflammatory polyps, inverted papillomas, and low-grade sinonasal adenocarcinoma are included in the list of clinical differential diagnoses for both REAH and COREAH (2,9). Glandular (seromucinous) hamartoma must be included in the differential diagnosis, as both lesions have similar clinical presentations and may overlap microscopically. Glandular hamartomas consist of an epithelial proliferation of mixed small and large glands with serous acini/tubules growing in clusters or lobules or randomly. The larger glands are usually lined by ciliated respiratory epithelium or attenuated flat cells, while the lining cells of the small glands are flat to cuboidal and may contain intra-cytoplasmic eosinophilic granules. Although REAH/COREAH and glandular hamartomas may all fall within the spectrum of sinonasal epithelial hamartomas, they are distinguished histologically by the presence of proliferating serous acini and the absence of chondro-osseous metaplasia (9).
REAH and COREAH can be distinguished from inflammatory polyps on both clinical and histological grounds. Clinically, most REAHs are solitary masses arising in the posterior nasal septum (2), whereas inflammatory polyps are bilateral and arise most commonly in the middle meatus. The presence of adenomatoid proliferation and stromal hyalinization are distinguishing histological features of REAH as opposed to the pauciglandular loose edematous stroma of inflammatory polyps.
Inverted papillomas are known to be locally aggressive tumors. Their clinical course, unlike that of REAH, is characterized by bone destruction, extension along mucosal surfaces, invasion into adjacent structures, local recurrence, and potential for malignant transformation (2). In addition, inverted papilloma has a known predilection for the lateral nasal wall, and only few arise from the septum (10). Histologically, the endophytically growing stratified squamous epithelium of inflammatory polyps is not seen in REAH.
REAH and low-grade sinonasal adenocarcinoma may have similar clinical presentations. However, histological distinction is rarely difficult unless the biopsy is exceptionally small. Features such as cribriform architecture, absence of ciliated epithelium lining the glands, desmoplastic stroma, and an elevated mitotic rate would strongly favor adenocarcinoma (8). Unlike inverted papilloma and sinonasal adenocarcinoma, REAH/COREAH is treated by choice by complete local excision (11). Cases followed up for up to 5 years showed no evidence of recurrence (2,11).
In conclusion, REAH/COREAH is an interesting although exceedingly rare benign hamartomatous lesion of the sinonasal area that may be clinically mistaken for more common, and potentially more aggressive, tumors of the head and neck. Awareness of this peculiar entity and its radiologic and pathologic characteristics is important to guide the clinician to an appropriately conservative and invariably curative surgical treatment.
REFERENCES1. Kumar V, Abbas AK, Fausto N. Neoplasia. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cortan pathological basis of human disease, 7th edition. Philadelphia, PA: Elsevier-Saunders Publishing Company; 2005. 272 p.
2. Wenig BM, Heffner DK. Respiratory epithelial adenomatoid hamartomas of the sinonasal tract and nasopharynx: a clinicopathologic study of 31 cases. Ann Otol Rhinol Laryngol. 1995;104:639-45.
3. Graeme-Cook F, Pilch BZ. Hamartomas of the nose and nasopharynx. Head Neck. 1992;14:321-27.
4. Adair CF, Thompson LDR, Wenig BM, Heffner DK. Chondoosseeous and respiratory epithelial hamartomas of the sinonasal tract and nasopharynx, Mod. Pathol. 1996;9(1):100.
5. Roffman E, Baredes S, Mirani N. Respiratory epithelial adenomatoid hamartomas and chondro-osseous respiratory epithelial adenomatoid hamartomas of the sinonasal tract: a case series and literature review. Am J Rhinol. 2006;20:596-90.
6. Flavin R, Russell J, Phelan E, McDermott MB. Chondroosseous respiratory epithelial adenomatoid hamartoma of the nasal cavity: a case report. Int J Pediatr Otorhinolaryngol. 2005;69:87-91.
7. Ozolek JA, Hunt JL. Tumor suppressor gene alterations in respiratory epithelial adenomatoid hamartoma (REAH): comparison to sinonasal adenocarcinoma and inflamed sinonasal mucosa. Am J Surg Pathol. 2006;30:1576-80
8. Sangoi AR, Berry G. Respiratory epithelial adenomatoid hamartoma: diagnostic pitfalls with emphasis on differential diagnosis. Adv Anat Pathol. 2007;14:11-6.
9. Perez-Ordoñez B. Hamartomas, papillomas and adenocarcinomas of the sinonasal tract and nasopharynx. J Clin Pathol. 2009;62:1085-1095.
10. Melroy CT, Senior BA. Benign sinonasal neoplasms: a focus on inverting papilloma. Otolaryngol Clin North Am 2006; 39:601-617
11. Endo R, Matsuda H, Takahashi M, et al. Respiratory epithelial adenomatoid hamartoma in the nasal cavity. Acta Otolaryngol. 2002;122:398-400
1) M.D. (Resident).
2) M.D. (Assistant Professor).
3) M.D. (Associate Professor).
Institution: Faysal A. Fedda, M.D.1 Fouad I. Boulos M.D.1 Alain N. Sabri M.D.2
1 Department of Pathology and Laboratory Medicine, American University of Beirut Medical Center, Lebanon.
2 Department of Otolaryngology Head and Neck Surgery, American University of Beirut Medical Center, Lebanon.
Mailing address: Dr. Alain Sabri, American University of Beirut, P.O. Box 11-0236, Riad El-Solh, 1107 2020 Beirut, Lebanon.
Article received on May 24, 2011. Article accepted on June 25, 2011.