INTRODUCTIONAdductor spasmodic dysphonia is a disorder characterized by involuntary contractions of the adductor muscles of the larynx during phonation, resulting in strained, strangled, or forced voice quality, as well as intermittent voice breaks (1,2). The use of botulinum toxin for the treatment of spasmodic dysphonia was described by Blitzer in 1986 (3). Since this report, botulinum toxin injection has been the treatment of choice for spasmodic dysphonia in various centers worldwide. Botulinum toxin injection is a minimally invasive method that provides excellent functional results. The toxin blocks presynaptic acetylcholine release at the neuromuscular junction, causing a partial and reversible chemodenervation leading to muscle weakness, which reduces spasms in patients with dystonia (4).
The most widely used method for injecting botulinum toxin into the thyroarytenoid (TA) muscle is electromyography-guided injection (4). At our facility, botulinum toxin is typically injected with a flexible needle, which is passed through the biopsy channel of a fiberoptic laryngoscope and guided by video-assisted endoscopy (2,5).
The reported side effects of botulinum toxin injection into the TA muscle include dysphagia, aspiration, and transient dyspnea, effects that vary in terms of incidence and severity (6,10). Another factor observed in clinical practice is a change in glottal configuration, including vocal fold bowing and incomplete glottal closure (with or without ventricular fold paralysis).
Studies in ophthalmology and gastroenterology have shown that paralysis of adjacent muscles is the most serious complication of the use of botulinum toxin and is probably caused by excessive diffusion of the toxin (11-13). This probably accounts for the wide variability in laryngeal function and configuration after injection of botulinum toxin into the TA muscle, given that the adjacent (non-targeted) muscles include the lateral cricoarytenoid (LCA) muscle and the ventricular portion of the TA muscle itself. Therefore, the objective of the present study was to evaluate the diffusion of aniline blue, a stain specific to muscle fibers, after its injection into the TA muscles of larynges excised from human cadavers.
METHODThis was an experimental study of 18 larynges that were excised from human cadavers. The larynges were obtained from the University of São Paulo School of Medicine, São Paulo Municipal Department of Death Certification, located in the city of São Paulo, Brazil. All larynges were removed within the first 24 h after death. We used 14 male larynges and 4 female larynges. All larynges were obtained from adult individuals of 30-87 years of age (mean age, 55.5 years). The cause of death was not taken into consideration, given that all larynges were macroscopically intact.
In the present study, we used aniline blue, a water-soluble stain that targets muscle fibers, at a concentration of 2%. The methodology employed was similar to that used for the injection of botulinum toxin in the Clinical Otolaryngology Department of the University of São Paulo School of Medicine Hospital das Clínicas (5). We used a flexible needle (model no. IN-2010; Machida Endoscope Co., Ltd., Tokyo, Japan) with a 0.5 mm tip. The needle was introduced into the TA muscle and middle third of the vibratory portion of the vocal fold, approximately 1 mm lateral to junction of the vibratory portion and the floor of the laryngeal vestibule (Figure 1). We injected 0.2 mL of aniline blue into the right and left vocal folds of each larynx. This volume (0.2 mL) is the volume that is used for botulinum toxin injections at our facility. The larynges remained in an anatomical position for 30 min, after which they were immersed in 10% buffered formalin and left for 7 days. The larynges were then placed in 7% nitric acid for 24 h for decalcification, being subsequently immersed in the same buffered formalin solution. The larynges were removed from the buffered formalin solution and stored at -10oC. In order to standardize the laryngeal sections, we drew a line (corresponding to the projection of the right vocal fold) on the ipsilateral thyroid cartilage lamina, parallel to the lower border of the cartilage. After measuring the width of the lamina along this line, we divided the line into 4 equal parts. The larynges were coronally sectioned with a microtome blade at 3 points (at the demarcated levels), resulting in 4 sections (designated sections I, II, III, and IV, from posterior to anterior). All sections were examined under a surgical microscope in order to identify the regions and muscles that showed aniline blue staining. For subsequent analyses, the sections were photographed with a digital camera (DSC-S30; Sony Corporation, Tokyo, Japan) at a resolution of 640 X 480 pixels.
We examined the TA, LCA, cricothyroid (CT), and posterior cricoarytenoid (PCA) muscles for aniline blue staining. We examined both sides of the sections because of the possibility of finding different degrees of aniline blue staining at different depths. Stained muscles were defined as those in which the entire length of the muscle (in at least one of the sections) showed aniline blue staining. Partially stained muscles were defined as those in which part of the muscle, however small, did not show staining. In order to analyze the PCA muscle, we dissected the posterior lamina of the cricoid cartilage.
We compared the genders in terms of dye diffusion to the muscles studied, each cadaver being considered as one case. Dye diffusion was defined as the presence of aniline blue staining on at least one side (right or left). We then measured the TA muscle in the section in which it was at its largest, using a ruler divided into millimeters. The TA muscle was measured in the lateromedial direction (width) and in the craniocaudal direction (height), as shown in Figure 2.
In order to compare the muscles in terms of dye diffusion, we used the McNemar's test for paired proportions. In order to compare the genders in terms of dye diffusion, we used Fisher's exact test. In order to determine whether TA muscle width and height had any influence on dye diffusion to non-injected muscles, we adjusted the logistic regression model, testing the parameters with the Wald test. In order to compare the genders in terms of laryngeal size, we used the nonparametric Mann-Whitney test. For all tests, the level of significance was set at 5%.
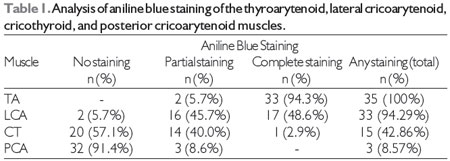
RESULTSWe evaluated 18 larynges (with 36 vocal folds). The left vocal fold of one of the larynges was damaged during the sectioning process, and the larynx was therefore excluded from the study sample. Thus, our final sample consisted of 35 hemi-larynges. Table 1 shows the analysis of aniline blue staining of the TA, LCA, CT, and PCA muscles.
Aniline blue staining was observed most often in the LCA muscle, followed by the CT and PCA muscles. We found statistically significant differences between the LCA and CT muscles and between the LCA and PCA muscles in terms of the frequency of dye diffusion (p < 0.00001). We also found a statistically significant (albeit weaker) difference between the CT and PCA muscles in terms of the frequency of dye diffusion (p < 0.0013).
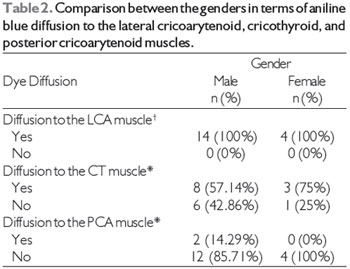
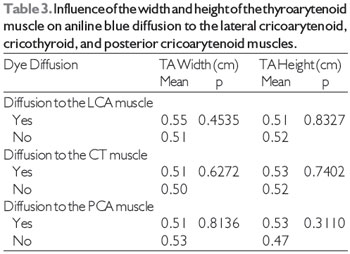
Table 2 shows the results of the comparison between the genders in terms of the frequency of dye diffusion. Since there was dye diffusion to the LCA muscle in all larynges, it was impossible to perform a statistical analysis of this variable for the LCA muscle. We found that neither the width nor the height of the TA muscle had a significant influence on aniline blue diffusion to the LCA, CT, and PCA muscles (Table 3).
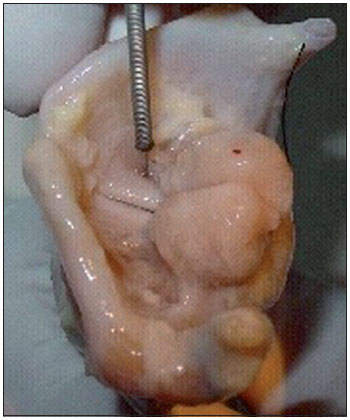
Figure 1. Introduction of the needle into the injection site.
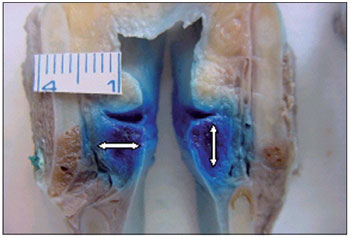
Figure 2. Points at which the width and height of the thyroarytenoid muscle were measured.
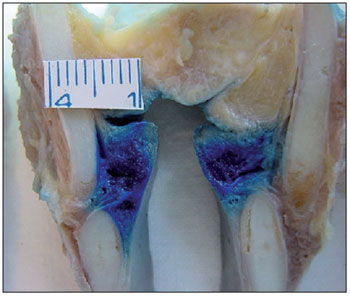
Figure 3. Lateral cricoarytenoid muscles showing aniline blue staining.
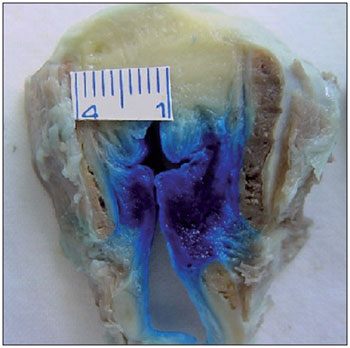
Figure 4. Cricothyroid muscle stained on the left.
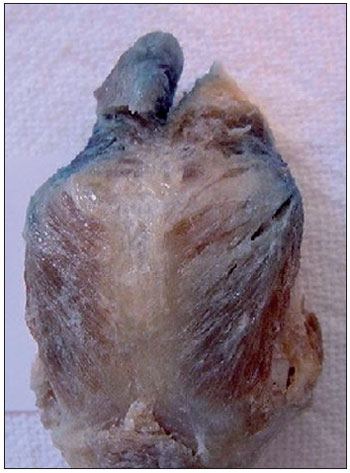
Figure 5. Posterior cricoarytenoid muscle partially stained on both sides.
Botulinum toxin injections into the TA muscle have been widely used in the treatment of spasmodic dysphonia. Botulinum toxin is produced by the anaerobic bacterium Clostridium botulinum, and botulinum toxin type A is the form that is used therapeutically. Botulinum toxin blocks presynaptic acetylcholine release at the neuromuscular junction, causing partial and reversible chemodenervation leading to muscle weakness, thereby reducing spasticity in patients with dystonia (4).
The most serious complication of botulinum toxin injection in the treatment of dystonia has been reported to be paralysis of muscles other than the target muscle, which is most likely attributable to toxin diffusion from the injected muscle to adjacent muscles. Various authors (14-16) have reported botulinum toxin diffusion to pharyngeal and laryngeal muscles after injection of the toxin into the sternocleidomastoid muscle for the treatment of spasmodic torticollis. According to these authors, the complications were dysphagia, hoarseness, and vocal fold paralysis. Ludlow et al (9) noted that, after injection of botulinum toxin into the TA muscle, there was transient dysphagia for liquids, breathiness, transient dyspnea, and aspiration, all of which were attributed to diffusion of the toxin to adjacent muscles.
The use of excised larynges allows the larynx to be cut into several sections after dye injection. However, an excised larynx has the disadvantage of being a devitalized tissue, which is immobilized (not subject to the motion that accompanies breathing, swallowing, and phonation) and without blood circulation or lymph flow. Therefore, it does not accurately reflect normal laryngeal function. In the excised larynges used in the current study, dye diffusion occurred passively, i.e., without the aid of circulation or active motion, and was probably due to hydrostatic pressure on the fluid-filled compartment of the muscle.
In the present study, the LCA muscle, which is the muscle that is closest to the TA muscle, was the most affected by diffusion (Figure 3). We found that 94.29% of the LCA muscles studied showed aniline blue staining. The LCA muscle is an important adductor muscle that positions the vocal processes along the midline through the rotation of the arytenoids. Therefore, LCA muscle involvement can lead to inadequate closure of the posterior third of the glottis, with incorrect positioning of the vocal process, leading to breathiness and aspiration.
Aniline blue diffusion to the CT muscle was observed in 42.86% of the cases (Figure 4). It is known that CT muscle involvement can lead to decreased fundamental frequency of phonation and, when accompanied by LCA and TA muscle paralysis, can decrease vocal intensity. The PCA muscle is the only abductor muscle of the larynx. In the present study, aniline blue diffusion to the PCA muscle was observed in 8.57% of the cases studied (Figure 5).
Although the risk of bilateral diffusion is small, it should be taken into consideration when botulinum toxin is used in the treatment of patients with spasmodic dysphonia, particularly when the toxin is injected bilaterally, because bilateral toxin diffusion can lead to a clinical situation that is similar to bilateral adductor vocal fold paralysis. Although it is a rare complication, bilateral paralysis following botulinum toxin injection has been reported in patients with adductor spasmodic dysphonia (17). Therefore, different degrees of muscle paralysis and paresis (in isolation or in combination) can explain the different laryngeal configurations observed in such patients.
Another parameter analyzed in our study was the influence of gender on aniline blue diffusion to non-injected muscles. This was tested for each muscle individually. For the LCA muscle, it was impossible to analyze this parameter because all of the larynges studied showed dye diffusion to the LCA muscle, with the exception of 2 hemi-larynges. With regards to the CT and PCA muscles, we found that gender had no statistically significant influence on dye diffusion to these muscles. Other authors have also found that there are no significant gender-related differences in diffusion (18).
In addition, we tested whether the height and width of the TA muscle influenced dye diffusion to non-injected muscles. We found that neither the height nor the width of the TA muscle had any influence on dye diffusion to any of the adjacent muscles studied.
Note: TA, thyroarytenoid; LCA, lateral cricoarytenoid; CT, cricothyroid; PCA, posterior cricoarytenoid.
Note: LCA, lateral cricoarytenoid; CT, cricothyroid; PCA, posterior cricoarytenoid
*p = 0.622 for the CT muscle; p = 1.000 for the PCA muscle.
+Since there was dye diffusion to the LCA muscle in all larynges, it was impossible to perform a statistical analysis for this muscle.
Note: TA, thyroarytenoid; LCA, lateral cricoarytenoid; CT, cricothyroid; PCA, posterior cricoarytenoid.
Based on the results of the present study, we can conclude that the probability of botulinum toxin diffusion to muscles that are adjacent to the TA muscle is nearly 100% for the LCA muscle and nearly 50% for the CT muscle. Albeit less common, diffusion of toxin to the PCA muscle can also occur. In addition, it seems that neither gender nor laryngeal size has any significant influence on the pattern of diffusion.
REFERENCES1. Behlau MS, Pontes PAL, Dedo HH. Spastic Dysphonia of the focal laryngeal dystonia: the evolution of the concept of the same disease. Acta AWHO. 1991;10(2):89-95.
2. Tsuji DH, Sennes LU, Pinho, SMR, Barbosa E. Technical application of botulinum toxin through the flexible endoscopel. In: Third Brazilian Congress from Laryngology and Voice, Annals. 1995; P 1.
3. Blitzer A, Brin MF, Fahn S, et al. Botulinum toxin (BOTOX) for the treatment of "Spastic Dysphonia"as part of a trial of toxin injections for the treatment of other cranial dystonias. Laryngoscope. 1986; 96:1300-1.
4. Evans CM, Williams RS, Shone, CC. Botulinum type B. Its purification, radioiodination and interaction with rat-brain synaptosomal membranes. Eur. J. Biochem.1986;154:409-16.
5. Tsuji DH, Sennes LU, Imamura R, Koishi HV. Technical Injection of Botulinum Toxin by flexible endoscopel. Arq Fund Otorrinolaringol. 2001;5(3):137-43.
6. Borodic GE, Joseph M, Fay L, Cozzolino D, Ferrante RJ. Botulinum A toxin for the treatment of spasmodic torticollis. Dysphagia and regional toxin spread. Otolaryngol. Head Neck Surg. 1990;12:392-8.
7. Shaari CM, George E, Wu BL, Biller HF, Sanders I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope. 1991;101:960-4.
8. Ludlow CL, Naunton RF, Sedory SE. Effects of Botulinum Toxin Injections on Speech in Adductor Spasmodic Dysphonia. Neurology. 1988;38:1220-5.
9. Ludlow CL, Bagley JA, Yin SG. A comparison of different injection techniques in the treatment of spasmodic dysphonia with botulinum toxin. J. Voice. 1992;6:380-6.
10. Brin MF, Blitzer A, Fahn S et al. Adductor Laryngeal Dystonia (Spastic Dysphonia): Treatment with Local Injections of Botulinum Toxin (BOTOX). Mov. Disord. 1989;4:287-96.
11. Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans. Am. Ophthalmol Soc. 1981;79:734-70.
12. Elston JS, Russel RW. Effects of Treatment with Botulinum Toxin on Neurogenic Blepharospasm. Br. Med. J. 1985;290:1857-9.
13. Hallan RI, Melling, J, Womack NR. Treatment of Anismus in Intractable Constipation with Botulinum A. Toxin. Lancet. 1988;2:714-7.
14. Stell R, Thompson PD, Marsden CD. Botulinum Toxin in Spasmodic Torticollis. J. Neurol. Neurosurg. Psychiatry. 1988;51:920-3.
15. Koay CE, Alun-Jones T. Pharyngeal Paralysis Due to Botulinum Toxin Injection. J Laryngol Otol. 1989;103:698-9.
16. Liu TC, Irish, JC, Adams SG, Durkin LC, Hunt EJ. Prospective Study of Pacients'Subjective Responses to Botulinum Toxin Injection for Spasmodic Dysphonia. J Otolaryngol. 1996;25(2):66-74.
17. Venkatesan NN, Johns MM, Hapner ER, DelGaudio JM. Abductor paralysis after botox injection for adductorspasmodic dysphonia. Laryngoscope. 2010 Jun;120(6):1177-80.
1) Otolaryngologist, PhD. Specialist in Otolaryngology, School of Medicine, University of São Paulo.
2) Otolaryngologist. Specialist in Otolaryngology, School of Medicine, University of São Paulo.
3) Otolaryngologist, PhD. Associate Doctor, Department of Otolaryngology, School of Medicine, University of São Paulo.
4) Otolaryngologist, PhD. Professor in the Department of Otolaryngology, School of Medicine, University of São Paulo.
Institution: Department of Otolaryngology, University of São Paulo School of Medicine. Av. Dr. Enéas de Carvalho Aguiar, 255, São Paulo / SP - Brazil - Zip Code: 05403-900. Mailing address: Azis Arruda Chagury - Av. Dr. Enéas de Carvalho Aguiar, 255 - Instituto Central - São Paulo / SP - Brazil - Zip Code: 05403-900 - Telephone: (+55 11) 2661-6286 - E-mail: azischagury@gmail.com
Article received on March 4th, 2013. Article accepted on April 8th, 2013.