INTRODUCTIONPatients treated with cisplatin can present hearing alteration. There are reports of tinnitus, hearing loss and alterations on physiology of the inner ear (1,2,3,4,5). The grade of severity depends on individual predisposition, dose, treatment period, administration passage, administration manner and age (6,7,8,9). Initial lesion occurs on cells of the spiral basal of cochlea, implicating external hair cells (10), causes sensorineural hearing loss usually symmetric one and affects high frequencies - 4,000 Hz to 8,000 Hz; and with dose accumulation it can progress to speech frequencies (1,11,12,13,14).
The presence of acoustic energy on inner ear in a spontaneous way or in response to sound stimuli was evidenced by KEMP (15). Such energy was named otoacoustic emissions. When these emissions are present, they represent an expressive sign of normal or almost normal cochlear function, becoming an indispensable tool for objective evaluation of sensorineural hearing imperfections (16), and so, acting on monitoring and on prevention of damages by ototoxic agents (17,18).
Distortion product arises from the inability of cochlea in enlarging two different stimuli in a linear way, causing an intermodulation that results in a distortion product (2f1-f2) (19,20,21). Through DPOAE, cochlear function can evaluated in a objective way and in small fractions, from basal spiral up to apical spiral, through frequencies variations of stimuli (16,22). The main advantage of this method is the specificity of frequency (23).
TARGETInvestigate hearing through conventional tonal audiometry, imitanciometry and otoacoustic emissions - distortion product in patients who made use of cisplatin and are cured of cancer.
MATERIAL AND METHODThis Project was approved by Research Ethics Committee - protocol 620/05.
Patients who participated in this study were the ones who used cisplatin and are cured of cancer, and searched Clínica de Fonoaudiologia da PUC- Campinas (Phonoaudiology Clinic) by themselves. 8 patients aging from 5 to 27 years were examined from March to July in 2006. These patients suffered from cancer from 6 months to 13 years old. 4 of them were men and 4 women. Patients who presented alteration on outer and middle ear were out of the research.
ProceduresIt was applied a questionnaire in order to collect information on presence or absence of hearing complaint, on otological history of patient and possible variables that might interfere on analysis of results on audiological evaluations: drug usage period, dose administration, age of beginning and ending of treatment.
Inspection on external acoustic meatus - it was verified the presence or absence of any obstacle to perform hearing evaluation.
Conventional Threshold Tonal Audiometry (AC 40 Interacoustcs) - detection of tonal threshold of hearing, through air pathway on frequencies 0,25;0,5;1;2;3;4;6;8 kHz. In the presence of hearing loss - threshold above 25dB (24,25), obtainment of tonal threshold through bone pathway, in a frequency interval of 0.5 to 4JHz.
Measures of acoustic immitance (AZ-7 Interacoustics) - on patients whose imitanciometry test revealed normal conditions on middle ear, it was done investigation with otoacoustic emissions.
Distortion product otoacoustic emissions (ILO292 - Otodynamics) - it was analyzed responses of DPOAE in the relation Signal/Noise, considering: present - responses from seven to nine frequencies; partial presence - responses from four to six frequencies and absent - resposes from zero to two frequencies. These categories were also used by CARVALHO, 2004 (20).
Statistical AnalysisIt was used statistical tests Kappa -PINHO, 2006 (26), accurate Fisher´s Test AGREST, 1990 (27); WOOLSON, 1987 (28) and Significance Test of Logistic Regression DAVID; LEMESHOW, 1989 (29).
RESULTSComparison between earsTable 2 shows result average of toanl threshold in dB NA, from right and left ear tests after therapy.
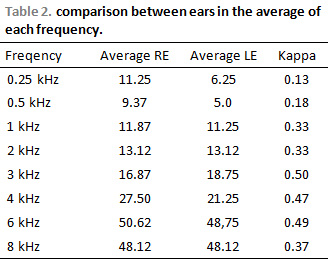
When value of Kappa is next to 1, difference is not considered. In general, values higher than 0.75 is considered next to 1.
There was a predominance of hearing loss to acute frequencies (4 kHz, 6 kHz, 8 kHz) on affected ears, according to Table 3.
Comparison of results obtained with distortion product and threshold tonal audiometry (tta)3.5 Evaluation of influence factorsFindings between right and left ears were divided as shown on Tables 5 and 6 according to possible interference of dose and age on audiometric resuts.
There is no difference between dose and age on patients with and without alteration. In order to verify age difference, it was used Accurate Fisher´s Test and to dose differences, it was used significance test of logistical regression.
According to p-value of the test (19.64%), there are no expressive differences between age with alterations and with no alterations.
According to p-value of Accurate Fisher´s Test, there are no expressive differences on alteration and no alteration between men and women.
DISCUSSIONAverages between right and left ear after therapy presented statistical difference (Kappa). There was predominance on hearing implication on right ear, although in some frequencies and in some patients hearing loss was asymmetric. Hearing losses were bilateral and symmetric types in 5 patiens (62.5%). Though, from the 16 evaluated ears, 10 of them (62.5%) presented alterations of hearing threshold. The studied showed that there was hearing implication in 62.5% of patients in conventional audiometry. The predominance of implication was in acute frequencies, but in the area that involves speech. Only 1 patient presented alteration on speech frequencies (1,2 kHz). These findings are in accordance with BENSADON, 1998 (1); DISHTCHEKENIAN et al, 2000 (11) ; GARCIA et al, 2003 (13) and KNIGHT et al, 2005 (14).
From the 8 patients, 3 of them presented tinnitus complaint. Although it is subjective symptom, it is not transitory. It is a possible finding in patients under ototoxic drug use (14). According to BENSADON,1998 (1), tinnitus, caused by cisplatin, is a transitory symptom for most patients. In studies by ZOCOLI et al, 2003 (4), nearly half of patients presented tinnitus up to the end of treatment.
Cisplatin dose used during therapy ranged between mg/m
2 and 780 mg/m
2, the average dose used in 8 patients was 362 mg/m
2. Chemotherapy period ranged from 4 months to 2 years. Chemotherapy doses were given in a interval from 1 to 9 weeks. Cisplatin dose was lower and more fragmented in 2 patients with no hearing alteration who were treated when still babies. There no statistical significance to drug dose. Despite that, findings agree with studies by DRESCHLER et al,989 (6); PODOSHIN, L et al,1989 (7); SAKAMOTO et al, 2000 (8); GODOFREDO,2001 (9) PAZ et al, 2000 (12), who attested that fragmented doses cause less damages than a single dose. Family predisposition and individual susceptibility can also lead to hearing loss. This information is also found SILVA et al ,2000 (18) e FENIMAN, 2001 (30).
Results from DPOAE agreed with findings on tonal audiometry as in AZEVEDO, 2003 (19).
Early diagnosis of hearing loss is important when revising applied therapeutic protocol in order to quality maintenance of patients´ communication, as well as hearing imperfection reduces its chances of occurrence in adulthood. Though, it is important to trace therapeutic protocols in multidisciplinary team in order to achieve healing with maximum quality of life.
CONCLUSIONThere was a prevalence regarding the affected side, the right one. Losses are symmetric in both ears. Hearing implication was predominant in frequencies above 6 kHz. There was an agreement between threshold tonal audiometry and distortion product otoacoustic emissions.
Protocol of audiological evaluation should be composed by interview, meatuscopy, conventional threshold tonal audiometry, high frequencies audiometry, measures of acoustic immitance and otoacoustic emissions during and after therapy in order to a proper measurement of grade and hearing affected moment.
In the same way that cisplatin, there are several other new chemotherapies that should be monitored. In this way, besides evaluating effectiveness, it is possible to evaluate cost-benefit and replacement by another equivalent.
ACKNOWLEDGEDoctors Maria José Mastellaro, and Rosana Ribeiro and Luis Henrique Chechinato Costa.
REFERENCES1. Bensadon RL. Estudo Clínico e Audiológico da Ototoxicidade da Cisplatina - comparação entre os exames de audiometria tonal convencional, audiometria de altas freqüências e emissões otoacústicas. São Paulo, 1998, p.100,(tese de Doutorado- Faculdade de Medicina - Otorrinolaringologia da Universidade de São Paulo).
2. Liberman PH, Schutz C, Pedalini ME, Gomes MV, Antonelli C. Monitorização auditiva em crianças portadoras de retinoblastoma: relato de 2 casos. Hospital do Câncer A.C. Camargo, 2002.
3. Bitencourt RF. Avaliação Audiológica em indivíduos expostos a agentes quimioterápicos. Paraná, 2003, p.138, (tese de Mestrado - Faculdade de Fonoaudiologia da Universidade Tuiuti).
4. Zocoli R, Reichow SL, Zocoli AMF. Emissões otoacústicas x Cisplatina: detecção precoce da ototoxicidade em pacientes oncológicos. Revista Brasileira de Otorrinolaringologia, 2003, 69:222-5.
5. Antunes LMG, Bianchi MLP. Antioxidantes da dieta como inibidores da nefrotoxicidade induzida pelo antitumoral cisplatina. Revista de Nutrição, 2004, 17:89-96.
6. Dreschler WA, Rjam VDH, Tange RA, Urbanus NAM. Role of high frequency audiometry in early detection of ototoxicity. Audiology, 1989, 28:211-20.
7. Podoshin L, Fradis M, Pavis JB. Ototoxicidade das gotas tópicas em pacientes com otite média crônica. Journal of Laryngology and Otology Israel, 1989.
8. Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg., 2000, 122(6):828-33.
9. Godofredo CB, Borges RHM, Baraúna GN. Ototoxicidade causada pela cisplatina em crianças. Estudo retrospectivo. Revista Brasileira de Otorrinolaringologia, 2001, 67:292-5.
10. Hyppolito MA et al. Otoproteção da amifostina aos efeitos ototóxicos da cisplatina: estudo em cobaias albinas por emissões otoacústicas produtos de distorção e microspia eletrônica de varredura. Revista Brasileira de Otorrinolaringologia, 2005, 71:168-73.
11. Dishtchekenian A et al. Monitorização Auditiva na Ototoxicidade. In: BARROS et al. Fonoaudiologia em Cancerologia. Fundação Oncocentro de São Paulo - Comitê de Fonoaudiologia em Cancerologia, 2000, p260-9.
12. Paz I, Codjambassis D, Pinto U. Emissiones Otoacústicas em la detección precoz de ototoxicidad inducida por cisplatino. Rev. Otorrinolaringol. CIR Cabez Cuello, 2000 (60):7-13.
13. Garcia AP, Iório MC, Petrilli AS. Monitoramento da audição de pacientes expostos à cisplatina. Revista Brasileira de Otorrinolaringologia, 2003, 69:215-21.
14. Knight KRG, Draemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology, 2005, 23(34):8588-96.
15. Kemp DT. Stimulates acoustic emissions from within the human auditory system. Journal of the Acoustical Society of America, 1978, 64:1386-91.
16. Lopes Filho O, Carlos RC. Emissões otoacústicas. In: Lopes Filho O, Rosimeire CC. Tratado de Fonoaudiologia. São Paulo: Rocca, 1997, p.221-37.
17. Vallejo JC, et al. Detecção precoce da ototoxicidade usando emissões otoacústicas produto de distorção. Revista Brasileira de Otorrinolaringologia, 2001, 67:845-51.
18. Silva MLG, et al. In: Silva MLG, et al. Quadros clínicos mais comuns. Vol. III. Cap. 14. Série Otoneurológica. São Paulo: Ateneu; 2000. p.119-130.
19. Azevedo MF. Emissões Otoacústicas. In: FIGUEIREDO, MS (org). Conhecimentos essenciais para entender bem Emissões Otoacústicas e BERA. São José dos Campos: Pulso, 2003, p.43-9.
20. Carvalho M. Limiares auditivos tonais em altas freqüências e emissões otoacústicas em portadores da desordem pigmentar do tipo vitiligo. São Paulo, 2004, p.114, (Tese de Mestrado - Faculdade de Medicina da Universidade de São Paulo).
21. Almeida EC, Araújo FCRS, Arroyo NL. Avaliação Audiológica do Recém-Nascido. In: Almeida EC, Modes LC. Leitura do Prontuário - Avaliação e Conduta Fonoaudiológica com o Recém-Nato de Risco. Rio de Janeiro: Revinter, 2005, p.72.
22. Munhoz MS, et al. Otoemissões Acústicas. In: Munhoz, et al. Audiologia Clínica. São Paulo: Editora Atheneu, 2000, p.121-48.
23. Molini E, Ricci G, Alunn I, Simoncelli C. Otoacoustic distortion produtcs in infants and adults: a comparative study. Acta Otorhinolaryngol Ital. 1998, 18(2):74-82.
24. Davis H, Silverman RS. Hearing and deafness. 3ª ed. New Cork: Holt, Rinehart & Wilson: 1970. p.253-79.
25. Silman S, Silvernan CA. Auditory diagnosis, principles and applications. London: Singular Publishing Group: 1991. p.215-32.
26. Pinho JS, et al. Métodos para estimação de reprodutividade de medidas. Índices: Estatística Kappa Disponível em URL: http://users.med.up.pt/joakim/
intromed/estatisticakappa.htm. Acesso em: 20 de junho de 2006.
27. Agresti A. Categorial Data Analysis John Wiley & Sons, 1990.
28. Woolson RF. Statistical Methods for the analysis of Biomedical Dat. ED. John Wiley & Sons, 1987.
29. David W, Lemeshow S. Applied Logistic Regression. John Wiley and Sons, 1989.
30. Feniman MR. Remédio: droga ou salvação. Jornal da USP - caderno opinião. 03 a 09 de set, 2001.
1. PhD in Education (Professor at Faculdade de Fonoaudiologia da PUC-Campinas -)
2. Graduation student of Phonoaudiology
3. Master student in Development Disturbances (Coordenator of Phonoaudiology Course at PUC-Campinas)
Pontifícia Universidade Católica de Campinas
address: Avenida Maria Martin Ottoboni, 210 - Condomínio Terras de Santana - Jardim Califórnia - Jacareí-SP CEP; 12306-700
This article was submitted to SGP - Sistema de Gestão de Publicações (Publication Management System) from RAIO on August 19, 2006 and was approved on September 8, 2006 13:30:26.