INTRODUCTIONThe (JNA) juvenile nasopharyngeal angiofibroma is a rare neoplasm, of benign histology, that affects almost exclusively male sex adolescent individuals. It corresponds to 0,05% of all head and neck tumors and is, however, the most common nasopharynx benign tumor (1,2). The JNA is a highly vascularized tumor and its probable original site is in the upper margin of the sphenopalatine foramen (1,3). Although it is classified as a benign tumor, the JNA is a locally aggressive tumor of high morbi-mortality and is associated to severe consequences, such as intracranial extension in 10% to 36% of the cases (4). The most solid basic knowledge regarding the JNA is pursuant to its histology.
The JNA is a tumor composed of star-shaped fibrocytes surrounded by a variable stroma of connective tissue. The tumor has thin wall vessels similar to lymphatic vessels that are an obligatory typical statement (1). Classically, the JNA may have an aspect similar to the erectile tissue; to a cavernous hemangioma with fibrous stroma or to a fibromatosis. (1). The central portions of the tumor are more fibrous with a minor number of vessels that fuse and form more expanded vessels. These central portions have the growth activity in a state of relative latency (5,6). Ulcerations are commonly found in the JNA periphery, the vascularization is more intense and is poor in terms of fibrous stroma. These peripheral regions represent the tumor active proliferation and the vessels assemble those of the granulation tissue (1,5). Such concentration in tissues suggest a gradient of maturation from the center to the periphery (5,6).
The JNA size relates to its histological content and a natural evolution occurs from a more vascular to a more fibrous tissue. The major tumors have a minor number of vessels and cells, major fibrous component and a tissue maturation level. In the other hand, the minor tumors have a central portion with abundant vessels in proliferation and capillary hyperplasia (6). The JNA vascular and fibrous components present a synchronic maturation, with regular and progressive change of blood vessels, and suggest that the tumor has a growth limited by the vascularization decreasing (5), with possible involution having been already reported (7). The noradrenergic fibers are located in the peripheral portions of the tumor, which may indicate that during surgical excision, keeping the dissection plan away from its parenchyma may decrease the bleeding by means of the local vasoconstrictor innervation maintenance (8).
The JNA histology has been closely related to the symptoms duration in the disease presentation. Nasal obstruction, tumor mass presence and facial deformity have been directly related to the tissue maturation and inversely to the cellularity (9). As far as epistaxis is concerned, its duration has shown a direct relation with the presence of vessels without muscular layer, which suggests that the JNA hemorrhagic characteristic is more related to its vessels fragility than to their number (9). Besides, it was observed that with the fibrous component development the bleeding trend decreases (9).
Classically the JNA histology has been analyzed based on the vascular and stromal component only. Wendler et. al characterize the tumor inflammatory component (10). A surprising number of mastocytes was found in the JNA (14.6% of the cells), in addition to lymphocytes, and they represent the predominant cellular types (10).
The JNA structure is duly characterized. However, there is a scarcity of studies that exploit their genetic and immunopathologic determiners, which would be crucial for the better understanding of this tumor etiology and pathogenesis. The present review is intended to exploit concisely the JNA genetic and molecular aspects, by remarking physiopathologic aspects that might contribute for future therapeutic approaches.
METHODA bibliographic survey has been carried out, without deadline, by using books, periodicals and studies accessed electronically at PBMED and LILACS database. All the references relevant to the theme found presented publication date between 1959 and 2007. The terms chosen for the research were: "Juvenile nasopharyngeal angiofibroma", "Juvenile nasoangiofibroma", "Genetics", " Morphology", "Growth factors", "Sexual hormones". "Juvenile nasopharyngeal angiofibroma", "Juvenile nasoangiofibroma", "Genetics", " Morphology", "Growth factors", "Sexual hormones".
RESULTS
Genetic factorsThe genetic determiners involved in the JNA pathogenesis remain unknown and important characteristics, such as preference for the male sex (Picture1A) and aggressive behavior (Picture1B and 1C) do not have genetic reason completely defined so far. However, the existence of a selectivity pursuant to sex, the spontaneous regression in some few patients and the malign transformation in very rare cases altogether suggest the existence of complex genetic mechanisms in its pathogenesis (11).
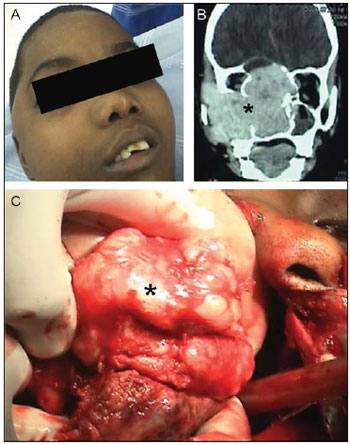
Picture 1. Nasopharyngeal angiofibroma topographic and surgical aspects. - (A) Facial deformity in young person with tumor in the preoperative. (B) Computed tomography: Coronal cut confirming intracranial invasion. (C) Intraoperative picture showing the tumor macroscopic aspect. The tumor is indicated in the pictures by asterisks (*).
The (GSTM1) glutationa-S-transferase M1 gene, constitutively expressed in the human cells and that has antioxidant cytoprotector properties, has its loss of expression associated to smoke and to the development of malignity in the upper respiratory passages tract. Gautham and collaborators investigated GSTM1 changes in the context of non-smoking patients with JNA. The results showed that three out of eight patients did not express the GSTM1, representing 37.5% of absence of this gene (12).
The growth factor similar to insulin II (IGFII) is a protein of stimulatory action for the growth, involved in the fetal development. The IGFII was detected with increased expression in 53% of the tumor tissues of patients with JNA. Such statement suggested that the IGFII gene may be involved in the JNA tumor obscure genesis (13). The IGFII gene expression is one of the targets of the early discovered genomic imprinting. The genomic imprinting is an alleles' selective inactivation process that results in transcriptional silencing. The IGFII gene undergoes maternal imprinting, that is, expresses normally only the paternal allele (14). The loss of imprinting leads to the abnormal biallelic expression that has been associated with the loss of the cellular cycle control. In a study investigating IGFII and H19 gene imprinting losses, a gene also related to the tumor pathogenesis, the IGFII biallelic expression was reported in 50% of the JNA studied cases. In addition to this, an IGFII and H19 overexpression was detected at important levels (15).
Pioneer recent studies, using the (CGH) comparative genomic hybridization, showed a number of chromosomal abnormalities in the JNA. Gains and losses were detected in autosomal chromosomes and, more frequently, in sexual chromosomes (16,17,18).
The changes quantity in number of copies is higher then that of several malign tumors, despite the JNA is histologically seen as a benign tumor. Losses in the genomic stability are possibly in connection to these statements and there are several consensual regions of chromosomal abnormalities to be evaluated. Amplifications detected in the AURKA and MDM2 genes, both relating to genomic instability, were consistent with the chromosomal alterations found (18).
The combination of the loss of chromosome Y with the gain of chromosome X is an association found in a significant way. As each chromosome X expresses almost invariably one gene for the (AR) androgen receptor, the gain of chromosome X results in gain of gene AR (17). Such gain may then explain the histological evidence documented in the JNA, of AR expression in 75% of the cases (19). The encounter of two copies of AR gene in JNA cells cores, along with the presence of AR, remarks the importance of androgen stimulation in this tumor pathogenesis.
Another recent genetic study using the CGH technique shows that in the JNA the genomic changes are characterized mostly by genic gains and not by losses. This is a possible indicator that the oncogenes activation is more important than the tumor suppressor genes inactivation (20). Specifically, genes of Rab via and proteins of connection to the retinoblastoma, among others, were found in several amplified regions and both of them have their overexpression associated to other tumors. However the relevance of such statements is not known. Another important aspect is the confirmation of differences in the genetic changes between primary and recurrent tumors. Such differences indicate these cases of recurrent tumors are actually of new tumors, because a standard of similar chromosomal aberrations would be expected in case the recurrent tumors were original from primary residual tumors (20).
A causal association has been suggested between the JNA and the (FAP) familiar adenomatous polyposis syndrome (21,22). This syndrome is a dominant autossomal condition characterized by several adenomas in the gastrointestinal tract, trend for the development of adenocarcinomas and extraintestinal manifestations. (23) JNA is supposed to be one of the FAP's extracolonic manifestations, with JNA occurring in a frequency up to 25 times higher in patients with this syndrome (21).
FAP results of germinative line mutations to the (APC) Adenomatous Polyposis Coli gene, locating in the chromosome 5q. This APC gene product, a tumor suppressor protein, is responsible for the β-catenine levels infra-regulation, among other factors. In a patient with FAP and JNA association, APC genes mutations were described, mainly in the tumor stromal cells. These data reinforce the suspicion that the JNA is one of the several FAP manifestations (24). Searching for explanations for the JNA pathogenesis also in the via APC/ β-catenine, an analysis was made of mutations involving such genes and their products in this tumor context. β-catenina intranuclear accumulation was detected in the major part of the JNA studied. Despite APC gene mutations were not found,
β-catenine gene high frequency mutations were found in most tumors (25). Some of these mutations are known to interfere with the β-catenine degradation, and therefore they may be involved in its intranuclear accumulation. (25,26,27). β-catenine is a signalizing protein that interacts with the androgenic signalization, connects selectively to the AR, and is translocated to the nucleus as part of this complex. Such translocation to the nucleus depends on the presence of 5α-dihydrotestosterone, and is a specific function of the AR. The β-catenine also acts as a coactivator, modulating the AR-dependent genes genic transcription.
Because it is only found in the stromal cells and not in endothelial cells or plain muscle cells, the β-catenine intranuclear accumulation suggests that this tumor key neoplasic element is the stromal component. In addition, in the same study, the genetic changes comparison in primary and recurrent tumors revealed identical mutations, which is against data mentioned previously. Such statements confirm the JNA definition as a real neoplasm (25,26).
p53 is a tumor suppressor gene and Her-2/neu is an oncogene that are located in the chromosome 17 and that also had their role in the JNA investigated. Both have changes in their expression, as for instance mutations in p53 gene and overexpression of Her-2/neu, relating to several tumors. p53, also called "molecular policeman" for its DNA lesion response actions, has undergone losses in 5 out of 7 JNAs analyzed, and no amplifications were noted in any case (28). The oncogene Her-2/neu, which appeared exercising independent stimulation over the AR in the prostate cancer context, had losses detected in similar quantities, without amplifications. However, such losses were not associated in a significant manner to the decreases in the mRNA levels to p53 or Her-2/neu, otherwise an increase was found in the levels of mRNA to p53 (28). It is possible that such incongruence is only apparent, because the interferences in the via of p53 by β-catenine are known. Taking into account the importance of β-catenine in the JNA, the p53 gene losses found may indicate a loss of its protecting function in this tumor context.
Another gene that has been investigated in the JNA is the prooncogene MYC, which has disorders in its expression as a cause for alteration in the proliferation, growth and cellular metabolism, and is associated to a large range of malignity. Heterogeneity was observed in advanced JNA, associated with C-MYC expression increase. The expression increase was qualified by increased levels of mRNA and by this protooncogene's protein (29). Therefore, the regulation above C-MYC may be connected with more aggressive growing phenotypes in the JNA. This is restated because interactions between C-MYC, β-catenine and AR were described in the neoplasic growth context in the prostate. β-catenine takes active part in the MYC prooncogene regulation and AR can connect to a responsive element in C-MYC. Thus, the C-MYC deregulation in the JNA is one more index in favor of the androgenic stimulation dependency this tumor has.
Mutations in ras family genes, Ki-ras and Ha-ras, already associated to smoking and several malign neoplasms, have not been connected to the JNA pathogenesis (30), nor to that of other head and neck tumors (31).
Molecular factorsToo little is known about the growth factors involved in the JNA pathogenesis, and its initiation and progression mechanisms are not very well known. However, their unique characteristics indicate that the growing factors involved are part of a complex interactions net, which includes cytokines, hormones and other mediators.
The (TGF-β) transformer of growth factor â is a polypeptide growth factor produced by many cellular types, such as fibroblasts, endothelial cells and macrophages. Its role in the neoplasic pathogenesis is complex, and exercises cellular cycle regulation functions, healing and angiogenesis induction. The TGF-β signalization path is associated to the aggressivity increase in many kinds of tumor (32). In a study using immunohistochemistry, the presence of TGF-b1 was shown activated in the nucleus, cytoplasm of stromal cells and capillary endothelium in 100% of 19 JNA's studied (33).
As already stated previously, a growth factor that seems to be involved in the JNA pathogenesis is the IGFII, for its significant overexpression in this tumor, along with other factors such as the (PDGF) platelet derivate growth factor, but without the same expression level (13). In the other hand, the IGF-1R, an IGF receptor associated to the tumorigenesis, was not detected in the JNA, and new studies are necessary in search of a better understanding of the real role of the IGF in the JNA (26).
A c-Kit and (NGF) nerve growth factor high pressure was detected by immunohistochemistry in the ANJ stromal cells, compared to nasal polyp tissues (26). High levels of c-Kit (CD117), a protein with tyrosine kinase action of the PDGFR (platelets derivate growth factor receptors) family, have already been related to other tumors. High levels of c-Kit found in the JNA make this protein a possible therapeutic target of tyrosine kinase specific inhibitors The other growth factor found in significant way, the NGF, seems to exercise influences in the angiogenesis, and in the differentiation and mastocytary function, which gains relevance considering the already reported large quantity of this cells in the tumor (10).
(FGFb) basic fibroblast growth factor was another mitogen factor detected in the JNA, and was associated with its pathogenesis (34). This factor has a stimulatory action known over the endothelial, muscular and fibroblast cells, and is implied in the processes of tissue remodeling, apoptosis, healing, angiogenesis and in the tumor growth. Like any other solid tumor, the JNA depends on the growth of vessels that offer intake to its proliferation and growth, which justifies the search for proangiogenic growth factors in its pathogenesis.
The high JNA vascularization, confirmed by immunohistochemistry, is directly related to the (VEGF) vascular endothelial growth factor. The VEGF is expressed specially in the stromal cells and JNA vessels and is associated to the proliferation and high vascular density of the tumor regions (35). However in this study no correlation was found between the tumor size and its vascularization. Therefore the VEGF expression in the JNA is connected to the larger vessels density, but not necessarily to their aggressivity (35). The larger vessels density in the JNA is also connected to the increased levels of other growth factors, such as bFGF and TGF-β, and to the VEGF receptors expression. These statements may help explain the excessive stromal growth peculiar characteristics and high JNA vessels density. In addition, the releasing inhibition and function of these growing factors is a possible therapeutic target for certain cases of JNA (36). In another large series, twenty four cases out or twenty seven had a VEGF significant marking, while the TGF-beta marking was found in fourteen cases and both could exercise a role in the tumor pathogenesis by the promotion of angiogenesis and cellular angiogenesis (37).
Another important confirmation in the study by Schuon et al. is that the Hif-1a expression, a molecule relating to the VEGF way and to the state of hypoxia, was shown in stromal cells and JNA vessels (36). This local hypoxia marker may indicate the presence of non-functional vessels. Therefore the high vessels density in the JNA does not mean necessarily a due oxygen tissue offer (36).
The JNA characteristic manifestation in male sex and young people, in a period of great hormonal influence, has always raised suspicion about the role of sexual hormones in its pathogenesis. Since an abnormality in the hypothalamic axis was proposed by Schif (38), as a JNA pathogenesis theory, the expression of hormonal receptors in the JNA has already been the purpose of many studies. Farag and his collaborators showed specific androgen receptors in the JNA with major affinity with dihydrotestosterone than with testosterone (39). In the JNA, anti-androgenic agents like flutamide may reduce the growth rate of the JNA
in vitro (40), and besides the androgen receptor, other steroid receptors have already been detected (39,41). However, no changes are found in the sexual hormones serum levels (39,42) nor changes in the JNA patients sexual maturity. More recently, the tumor definition as dependent on androgenic has been questioned (43), which can help explain the unconcluded results and the unpredictability of treatment with anti-androgenic (44,45). In spite of the existing controversy, the hormonal stimulation means remains a JNA therapeutic possible target. New clinical trials are required so that the real relevance of this approach is defined (46).
DISCUSSIONThe extensive knowledge about the pathology of JNA began to be established some decades ago, with the description of classical morphologic statements. The tumor histology and microstructure have been more recently clarified (see general aspects of microscopy in Picture 2). Despite the advances in the field of anatomopathologic description, there are a few studies concerning the JNA molecular and genetic aspects. Most investigations about the JNA genetics leads to results unconcluded or that do not add too much information to the existing knowledge about the tumor. The nuclear accumulation of β-catenine, an androgenic receptor coactivator, may be responsible for the almost exclusive male sex selectivity. This explanation is consistent with the statements of hormones serum levels in patients with JNA.
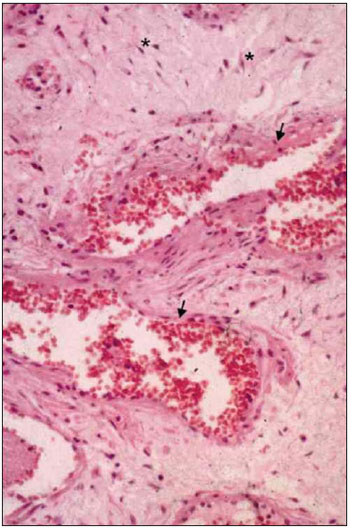
Picture 2. Nasopharyngeal Angiofibroma Histology. - The tumor classical aspects reveal star-shaped fibrocytes (*) in conjunctive tissue stroma, in addition to thin wall vessels (arrows).
For the growth and metastasis of solid tumors, angiogenic growth factors are essential. The JNA development seems to be influenced by VEGF, TGF-b1and IGFII, but the real production stimulus of these mediators have not yet been clearly defined. The possible therapeutic consequences of these statements are also unknown.
To theory about the JNA pathogenesis could have explained if the initial event of its development occurs in the endothelium or in the tumor stroma. Therefore, there are additional challenges in the JNA immunopathogenesis understanding, such as the phenotypic study of
in situ antitumoral immune response. The genetic changes study in primary tumors is necessary through "high-throughput" techniques with correlate genes grouping real time expression analysis for the detection of possible recurrence increased risk predictors. Such predictors will be able to help in the patients follow-up after surgical removal.
FINAL CONSIDERATIONSThere are several controversies regarding the JNA pathogenesis. However, even partially, genetic and molecular factors may explain the tumor aggressive and angiogenic characteristic in addition to its sex-related selectivity.
BIBLIOGRAPHIC REFERENCE1. Batsakis JG. Tumors of the head and neck: clinical and pathological considerations. 2a ed. Baltimore, MD: Williams & Wilkins; 1979, pp. 296-300.
2. Biswas D, Saha S, Bera SP. Relative distribution of the tumours of ear, nose and throat in the paediatric patients. Int J Pediatr Otorhinolaryngol. 2007, 71(5):801-5.
3. Beham A, Beham-Schmid C, Regauer S, Auböck L, Stammberger H. Nasopharyngeal angiofibroma: true neoplasm or vascular malformation? Adv Anat Pathol. 2000, 7:36-46.
4. Cummings C. Otolaryngology - Head and neck surgery. 4ª ed. 2005, pp. 1669-1672.
5. Liang J, Yi Z, Lianq P. The nature of juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 2000, 123:475-81.
6. Sennes LU, Sanchez TG, Bernardi FC, Saldiva PH. Tissue maturation during the growth of juvenile nasopharyngeal angiofibroma. Ann Otol Rhinol Laryngol. 2004, 113(1):34-38.
7. Weprin LS, Seimens PT. Spontaneuos regression of juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head and Neck Surg. 1991, 117:796-799.
8. Wang HW, Su WY, Wang JY. Noradrenergic innervation of juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol. 1994, 251: 123-126.
9. Sennes LU, Fortes FSG, Butugan O, Saldiva PH, Bernardi FC. Tissue maturation correlating to clinical manifestations in juvenile angiofibroma. Ann Otol, Rhinol Laryngol. 2005, 114(9):705-708.
10. Wendler O, Schäfer R, Schick B. Mast cells and T-lymphocytes in juvenile angiofibromas. Eur Arch Otorhinolaryngol. 2007, 264:769-775.
11. Makek MS, Fisch U, Andrews JC. Malignant transformation of a nasopharyngeal angiofibroma. Laryngoscope. 1989, 99:1088-1092.
12. Gautham K, Ogale SB, Shraddha RU, Ajay D. Expression of GSTM1 in angiofibromas. J Laryngol Otol. 2002, 116:352-354.
13. Nagai MA, Butugan O, Logullo A, Brentani MM. Expression of growth factors, protooncogenes and p53 in nasopharyngeal angiofibromas. Laryngoscope. 1996, 106(2):190-195.
14. Falls JG, Pulford DJ, Wylie AA, Jirtle RL. Genomic imprinting: implications for human disease. Am J Pathol. 1999, 154(3):635-647.
15. Coutinho-Camillo M, Brentani MM, Butugan O, Torloni H, Nagai MA. Relaxation of imprinting of IGFII gene in juvenile nasopharyngeal angiofibromas. Diagn Mol Pathol. 2003, 12:57-62.
16. Schick B, Brunner C, Praetorius M, Plinkert PK, Urbschat S. First evidence of genetic imbalances in angiofibromas. Laryngoscope. 2002, 112(2):397-401.
17. Schick B, Rippel C, Brunner C, Jung V, Plinkert PK, Urbschat S. Numerical sex chromosome aberrations in juvenile angiofibromas: genetic evidence for an androgen-dependent tumor? Oncol Rep. 2003, 10(5):1251-1255.
18. Schick B, Wemmert S, Bechtel U, Nicolai P, Hofmann T, Golabek W et al. Comprehensive genomic analysis identifies MDM2 and AURKA as novel amplified genes in juvenile angiofibromas. Head Neck. 2007, 29:479-487.
19. Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Mod Pathol. 1998, 11:1122-1126.
20. Heinrich U, Brieger J, Gosepath J, Wierzbicka M, Sokolov M, Roth Y. Frequent chromosomal gains in recurrent juvenile nasopharyngeal angiofibroma. Cancer Genet Cytogenet. 2007, 175:138-143.
21. Giardiello FM, Hamilton SR, Krush AJ, Offerhaus JA, Booker SV, Petersen GM. Nasopharyngeal angiofibroma in patients with familial adenomatous polyposis. Gastroenterology. 1993, 105(5):1550-1552.
22. Ferouz AS, Mohr RM, Paul P. Juvenile nasopharyngeal angiofibroma and familial adenomatous polyposis: an association? Otol Head Neck Surg. 1995, 113:435-439.
23. Gardner EJ. Follow-up study of a family group exhibiting dominant inheritance for a syndrome including intestinal polyps, osteomas, fibromas, and epidermal cysts. Am J Hum Genet. 1962, 14:376-390.
24. Curia M C, G Aceto, S Veschi, R Valanzano, L De Lellis. Genetic evidence that juvenile nasopharyngeal angiofibroma is an integral FAP tumour. Gut. 2005, 54:1045-1050.
25. Abraham SC, Montgomery EA, Giardiello FM, Wu TT. Frequent b-catenin mutations in juvenile nasopharyngeal angiofibromas. Am J Pathol. 2001, 158:1073-1078.
26. Zhang PJ, Weber R, Liang HH, Pasha TL, LiVolsi VA. Growth factors and receptors in juvenile nasopharyngeal angiofibroma and nasal polyps: an immunohistological study. Arch Pathol Lab Med. 2003, 127:1480-1484.
27. Rippel C, Plinkert PK, Schick B. Expression of members of the cadherin-/catenin-protein family in juvenile angiofibromas. Laryngo Rhino Otol. 2003, 82:353-357.
28. Schick B, Veldung B, Wemmert S, Jung V, Montenarh M, Meese E, et al. p53 and Her-2/neu in juvenile angiofibromas. Oncology reports. 2005, 13:453-457.
29. Schick B, Wemmert S, Jung V, Steudel W-I, Montenarh M, Urbschat S. Genetic heterogeneity of the MYC oncogene in advanced juvenile angiofibromas. Cancer Genet Cytogenet. 2006, 164:25-31.
30. Coutinho CM, Bassini AS, Gutierrez LG, Butugan O, Kowalski LP, Brentani MM, et al. Genetic alterations in Ki-ras and Ha-ras genes in juvenile nasopharyngeal angiofibromas and head and neck cancer. Sao Paulo Med J. 1999, 117:113-120.
31. Yarbrough WG, Shores C, Witsel DL, Weinsler MC, Fidler ME, Gilmer TM. Ras mutations and expression in head and neck squamous cell carcinomas. Laryngoscope. 1994, 104(11):1337-1347.
32. Kaklamani VG, Pasche B. Role of TGF-beta in cancer and the potential for therapy and prevention. Expert Rev Anticancer Ther. 2004, 4(4):649-61.
33. Dillard DG, Cohen C, Muller S, Del Gaudio J, Reichman O, Parrish B, et al. Immunolocalization of activated transforming growth factor beta 1 in juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg. 2000, 126:723-725.
34. Schiff M, Gonzalez AM, Ong M, Baird A. Juvenile nasopharyngeal angiofibroma contain a angiogenic growth factor: basic FGF. Laryngoscope. 1992, 102(8):940-945.
35. Brieger J, Wierzbicka M, Sokolov M, Roth Y, Szyfter W, Mann WJ. Vessel density, proliferation, and immunol immunolocalization of vascular endothelial growth factor in juvenile nasopharyngeal angiofbromas. Arch Otolaryngol Head Neck Surg. 2004, 130(6):727-731.
36. Schuon R, Brieger J, Heinrich Ulf R, Roth Y, Szyfter W, Mann Wj. Immunohistochemical analysis of growth mechanisms in juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol. 2007, 264:389-394.
37. Saylam G, Yucel OT, Sungur A, Onerci M. Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol. 2006, 70:227-234.
38. Schif M. Juvenile nasopharyngeal angiofibroma: a theory of pathogenesis. Laryngoscope. 1959, 84:2181-2194.
39. Farag MM, Ghanimah SE, Ragaie A, Saleem TH. Hormonal receptors in juvenile nasopharyngeal angiofibroma. Laryngoscope. 1987, 97(2):208-11.
40. Hagen R, Romalo G, Schwab B, Hoppe F, Schweikert HU. Juvenile nasopharyngeal angiofibroma: androgen receptors and their significance for tumor growth. Laryngoscope. 1994, 104(9):1125-1129.
41. Brentani MM, Butugan O, Oshima CT, Torloni H, Paiva LJ. Multiple steroid receptors in nasopharyngeal angiofibromas. Laryngoscope. 1989, 99(4):398-401.
42. Johns ME, MacLeod RM, Cantrell RW. Estrogen receptors in nasopharyngeal angiofibromas. Laryngoscope. 1980, 90:628-634.
43. Gatalica Z. Immunohistochemical analysis of steroid hormone receptors in nasopharyngeal angiofibromas. Cancer Lett. 1998, 127:89-93.
44. Gates GA, Rice DH, Koopmann CF Jr, Schuller DE. Flutamide-induced regression of angiofibroma. Laryngoscope. 1992, 102(6):641-644.
45. Labra A, Chavolla-Magana R, Lopez-Ugalde A, Alanis-Calderon J, Huerta-Delgado A. Flutamide as a preoperative treatment in juvenile angiofibroma (JA) with intracranial invasion: report of 7 cases. Otolaryngol Head Neck Surg. 2004, 130(4):466-469.
46. Montag AG, Tretiakova M, Richardson M. Steroid hormone receptor expression in nasopharyngeal angiofibromas. Consistent expression of estrogen receptor beta. Am J Clin Pathol. 2006, 125(6):832-7.
1. Doctor's Degree in Otorhinolaryngology by FMUSP. Head of Santa Casa Bahia's Otorhinolaryngology Service.
2. Academic of the Universidade Federal da Bahia's Medicine College. Student of scientific initiation of the Centro de Pesquisas Gonçalo Moniz, FIOCRUZ-BA.
3. Academic of the Universidade Federal da Bahia's Medicine College. Academic of the Universidade Federal da Bahia's Medicine College.
4. In course for Doctor's Degree in Pathology by the Centro de Pesquisas Gonçalo Moniz, FIOCRUZ-BA. Otorhinolaryngologist medical doctor of the Hospital Santa Izabel's otorhinolaryngology service, Salvador-BA.
5. In course for Doctor's Degree in Pathology by the Centro de Pesquisas Gonçalo Moniz, FIOCRUZ-BA. Medical doctor of the Hospital Universitário Professor Edgard Santos' Immunology service, Universidade Federal da Bahia, Professor of Medical Immunology of the Universidade Federal da Bahia's College.
Institution: Otorhinolaryngology Service, Santa Casa de Misericórdia da Bahia - Hospital Santa Izabel. Salvador / BA - Brazil.
Mail address:
Nilvano Alves de Andrade
Praça Conselheiro Almeida Couto, 500 - Nazaré
Salvador / BA - Brazil - Zipcode: 40050-410
E-mail: nilvano@gmail.com
Article received on May 18, 2008.
Article approved on July 31, 2008.