INTRODUCTIONThe human larynx study, mainly concerning the development and improvement of surgical techniques, is basic to meet the therapeutic needs. However, when achieved
in vivo this study becomes limited, since sometimes the surgery may bring phonatory results worse than the own pathology. Therefore, for laryngology study and research animal models are commonly used (1, 2). The animal experimentation may also contribute largely for this evolution, mainly for the easy access compared to human larynxes and for they are more easily controlled.
Some works researched histological and morphological characteristics of the larynx of some animals that made surgical techniques feasible (3). The animals models normally used for studies relating to operative techniques are pigs (3), dogs (4-8), rabbits (9) and cats (10).
The objective of this work is to analyze the laryngofissure with vocal cords grafts as an experimental surgical technique in male adult rabbits in order to contribute with data for the future development of other research projects.
METHODAfter approval of the research by the Research Project Analysis Commission (CAPPesq) of the Clinical Board of the Clinical Hospital of the Medicine College of the University of São Paulo (research protocols 200/02, 796/06), from March 2002 through April 2007, 46 New Zealand albino rabbits all healthy and of the male sex with corporal mass between 2.5kg and 4.0kg. We studied 46 New Zealand albino rabbits submitted to microsurgery in both vocal cords with autologous unilateral or bilateral graft of fat or fascia.
The surgical procedures and the immediate postoperative cares were carried out entirely in the Medical Research Laboratory of Otorhinolaryngology (LIM-32) of the Medicine College of the University of São Paulo (FMUSP). After surgery the animals were sent to the FMUSP's animal room and Granja RG animal room, located in the city of Suzano, where they remained with daily feeding cares and submitted to room temperature controlled until they were sacrificed with intravenous injection of 2.0 mL of potassium chloride (KCL) at 20%, after anesthesia according to the abovementioned protocol and time previously scheduled.
The animals received intramuscular anesthesia with Xilazin (5mg/kg) associated to Ketamine (25mg/kg). They were kept in spontaneous ventilation without need for tracheal cannula and fixed to the surgical table by the four paws; and the front paws were maintained along the body. Even during the larynx manipulation (laryngofissure and its closure) it was not necessary to use tracheal cannula.
We performed tricotomy in the anterior cervical region which expanded from the jaw to the wishbone antisepsis with PVP-I dyeing at 10%. The rabbits were submitted to cervical incision in the middle line, from the upper margin of the thyroid cartilage up to the lower cricoid cartilage, with lamina bistoury 15, on the skin and subcutaneous, to expose the thyroid and cricoid cartilage (Picture 1). There was no need for hemostasis in this phase of the procedure. With the help of surgical microscope (model Inami L 860, lens of 200 mm, angulated binocular, ocular of 12.5 x, lighting by optic fiber and halogen light source of 15V and 150W), the cricothyroid membrane was incised in the middle line, with lamina bistoury 12, that allowed for a view of the subglottis. It was eventually necessary to use delicate cautery in this phase of the procedure. The thyroid cartilage was then also incised with lamina bistoury 12 at its frontal middle line, up to about the lower half of the thyroid cartilage, which allowed for the exposure of the vocal cords (Picture 2). Both vocal cords were submitted to longitudinal incision of 1mm of length at 0.5mm of the freeboard. We carried out a delicate displacement of the mucosa along the medial margin also expanding towards the lower portion of the subglottis, with a Hollemback stiletto 3s, employed in dentistry. Therefore, we made a bag in the vocal cord longitudinal axis direction, and used the thyroarytenoid muscle as a main reference. The graft (muscular fascia or fat), with about 2mm of diameter and 1mm of height (measured with slide gauge), was positioned in such bag dissected between the unilateral and bilateral thyroarytenoid muscle. The fat was removed from the own cervical incision for access to the larynx and the muscular fascia (fascia tin) was removed after incision and dissection in the thigh antero-lateral region (Picture 3). The vocal cord incision was not sutured. The closure of the thyroid cartilage, the cricothyroid membrane and the skin was initially made (up to rabbit number 8) with Catgut 4-0 sutures; with separate points in only two plans (larynx and skin). From rabbit number 9 we used 5-0 Prolene thread, in continuous suture, for closure of the thyroid cartilage and cricothyroid membrane incision. Then, the pre-laryngeal musculature was sutured with Catgut 3-0, as well as the skin (in separate plans), both in continuous suture (Picture 4). All operated animals were kept alive with daily cares and healing process control. The rabbits received antibiotic therapy with procaine Benzilpenicilin 300000 UI, potassium Benzilpenicilin 100000 UI, diluted in 3 mL of distilled water, and applied intramuscularly 0.4 mL per dose. The first dose was applied during the procedure and two others every 24 hours.
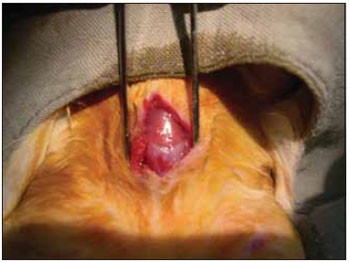
Figure 1. Exposure of the thyroid cartilage.
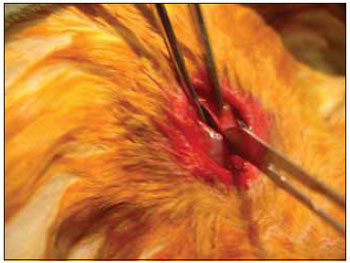
Figure 2. Exposure of the vocal cords.
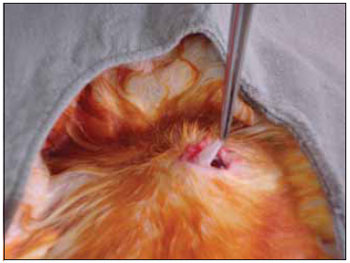
Figure 3. Dissection in the thigh antero-lateral region.
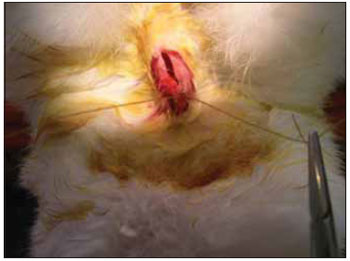
Figure 4. Suture of the pre-laryngeal muscles.
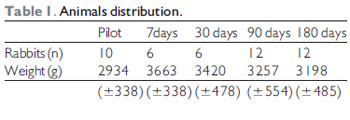
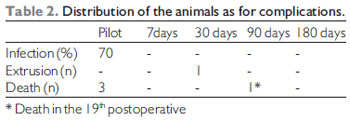
The weight average among the animals was of 3200 grams (Table 1) and they were all of the same sex (male). Table 2 describes the animals distribution regarding the complications: there were 4 losses of 3 animals (rabbits 1, 2 and 5) until the first week of the postoperative period and another (rabbit 12) after 19 days after surgery. The animals cause of death was ascribed to respiratory discomfort associated to cervical infection (3 animals) and urinary infection (rabbit 12). Until the eighth animal operated, still in the pilot study, there was formation of abscess under operative injury, which communicated to the trachea through the cricothyroid membrane, which was partially destroyed by the infection. Such fact was probably due to the cricothyroid membrane suture dehiscence that had been sutured with Catgut 4-0. From these data, we modified the suture (as described) and there was no infection, hematoma or dehiscence of the subsequent animals sutures. We had a case of graft extrusion (rabbit 34 - muscular fascia). In the Pilot Group rabbits, in which we identified the cervical infection, the grafts remained in the programmed position.
From the descriptions by Hirano (11) of the vocal cord structure in layers and its association with the vibratory standard, the laryngology transformed its concepts and enables a better understanding of the vocal alterations. The body and coverage theory by Hirano and Kakita (12), brought a basic understanding of the phonation physiology and changed the surgical concepts philosophy, and experimental works allowed the test of new techniques.
In spite of the availability of several experimentation animals, which would be the best experimental model for laryngologic studies? Kurita et al. (13) reported that the pig's vocal cord histology, as well as the larynx dimensions, are mostly similar to the human ones. According to the same author, this does not indicate that such animal is necessarily the best model for phonation studies, because factors such as intrinsic muscle of the larynx, and others, must be considered. According to Garret et al. (3), based on histological and stroboscopic aspects, the dog is the model that best approaches the ideal. In the other hand, the rabbit is a docile animal, of easy anesthetic management, that is accommodated in animal rooms common cages and is largely used in models for immunologic response study (14).
Upon analysis of the histology of the proper lamina and the thyroarytenoid muscle of the rabbits, we noted its similarity with the human vocal cord (13), which confirms the division in layers and the presence of collagen and extracellular matrix cells.
In this work, the method used to anesthetize the rabbits allowed us to access the vocal cords through laryngofissure with spontaneous ventilation, which favored the delicate and safe manipulation of the vocal cords and grafts, without any undesirable damage to any structure.
The dissection of the proper lamina and the thyroarytenoid muscle was carried out by using the surgical microscope. As the proper lamina of the rabbit is very thin and adhered to the epithelium (12), we opted to produce a bag between the proper lamina and the thyroarytenoid muscle in order to reduce the possibility of a rupture to the piece and extrusion of the graft. In human beings, Sataloff et al (15) were pioneer to describe the creation of a bag in the Reinke's space, between the epithelium and the vocal link, followed by the filling of fat injected with Bruning's syringe and a thick needle to prevent trauma and the block destruction.
For being a histological study and aiming at observing the graft incorporation, the external access and the graft insertion between the proper lamina and the muscle didn't cause any inconvenience. If the study was focused on the vibratory features after the grafting, the most loyal methodology would be a direct laryngoscopy and positioning of the graft in the most superficial layers of the proper lamina, that is, right under the epithelium.
CONCLUSIONThe study enables the conclusion that the experimental laryngofissure in rabbits is a safe method that may be used for laryngological studies.
BIBLIOGRAPHICAL REFERENCES1. Isshiki N, Morita H, Okamura H, Hiramoto M. Thyroplasty as a new phonosurgical technique. Acta Otolaryngol. 1974, 78:451-457.
2. Carneiro CG, Sennes LU, Saldiva PH, Tsuji DH, Ximenes Filho JÁ. Assesment of collagen deposits after implant of fascia lata and fat in the vocal fold of rabbits: histomorphometric study. Rev Bras Otorrinolaringol. 2005, 71:798-802.
3. Garret CG, Coleman JR, Reinish L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000, 110:814-824.
4. Wexler DB, Gray S, Jiang J, Titze I. Phonosurgical studies: fat-graft reconstruction of injured canine vocal cords. Ann Otol Rhinol Laryngol. 1989, 98:668-673.
5. Hill DP, Meyers AD, Harris J. Autologous fat injection for vocal cord medialization in the canine larynx. Laryngoscope. 1991, 101:344-48.
6. Mikus JL, Kilpatrick SE, Koufman JA. Fate of liposuctioned and purified autologous fat injections in the canine vocal fold. Laryngoscope. 1995, 105:17-22.
7. Woo P, Rahbar R, Wang Z. Fat implantation into Reinke space: A histological and stroboscopic study in the canine. Ann Otol Rhinol Laryngol. 1999, 108:738-744.
8. Duke SG, Salmon S, Blalock D, Postma GN, Koufman JA. Fascia augmentation of the vocal fold: graft yield in the canine and preliminary clinical experience. Laryngoscope. 2001, 111:759-764
9. Duprat AC, Costa HO, Almeida RR, Lancelotti C, Caron R. Histologic behavior of the inflamattory process in autologous fat implantation in rabbit vocal folds. Ann Otol Rhinol Laryngol. 2004, 111:636-640.
10. Saccogna PW, Werning J, Setrekian S, Struss M. Lipoinjection in paralysed feline vocal fold: Study of graft survival. Otolaryngol Head and Neck Surg. 1997, 117(5):465-70.
11. Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr. 1974, 26:89-94.
12. Hirano M, Kakita Y. Cover-body theory of vocal fold vibration, In: Daniloff RG, editor. Speech science: recente advances. San Diego: College-Hill Press; 1985, pp.1-46.
13. Kurita S, Nagata K, Hirano M. Comparative study of the layer structure of the vocal fold. In: Bless, DM. Vocal fold physiology. 1st ed. San Diego: Singular; 1995, pp.03-21.
14. Harlow E, Lane D. Antibodies: a laboratory model. Cold Spring Harbour Laboratory. 1988, 92-111.
15. Sattaloff RT, Spiegel JR, Hawkshaw M, Rosen DC, Heuer RJ. Autologous Fat implantation for vocal fold scar: A Preliminary Report J Voice. 1997, 11(2):238-246.
1. Doctor at FMUSP. Otorhinolaryngologist.
2. Doctoral Degree in ORL at FMUSP. Otorhinolaryngologist.
Institution: Departamento de Otorrinolaringologia da FMUSP. São Paulo / SP - Brazil
Mail address:
Christiano de Giacomo Carneiro Alameda
Rua Ministro Rocha Azevedo, 644/144 - Cerqueira César
São Paulo / SP - Brazil - Zip code: 01410-000
Telephone (+55 11) 8143-6600
E-mail: fabricioorl@yahoo.com.br
Article received on June 03 2008.
Approved on May 31 2009.