INTRODUCTION The profound deafness is a disability that directly affects the individual and the people around you, since it can affect their communication, personality and relationships with others (1).
The use of individual hearing aids (HA) may benefit individuals with hearing impairment of varying degrees (from mild to profound). However, as a sound amplifier, you need a cochlear reserve enough that there might be a good understanding of the sound, and especially speech. Some people, however, present a very important hearing dysfunction, and cannot get benefits with the use of hearing aids. These individuals can then be candidates for cochlear implant (1,2). Cochlear implants are traditionally indicated for patients whose hearing loss does not allow a functional gain sufficient speech perception with conventional hearing aids (3).
The cochlear implant (CI) is a system able to functionally restore the auditory system. (1.4). Since the launch of Nucleus 22 multichannel CI in 1978 by GRAEME CLARK (5) there is a concern of increasing technology. Among the different brands of multichannel cochlear implants, are models Nucleus 22 (N22) and Nucleus 24 (N24).
The N22 is a multichannel cochlear implant consists of an internal component (composed of receiver / stimulator, and beam with 22 electrodes), the Spectra speech processor model 22, and the transmitting antenna. The speech processor has a function to convert the information transmitted by the microphone into electrical signals that represent aspects of speech that might be perceived by the patient. Each model has a cochlear implant speech processing strategy for achieving this goal (6,7).
In N22, the speech processing strategy used is the Spectral Peak (SPEAK). This selects an average frequency of 6-8 from a filter 20 frequency bands. These selected frequencies, designated maximum, are presented to the electrodes in a non-simultaneous stimulation to an average speed of 250 Hz to minimize interactions between the electrodes (6).
With advances in technology, the Nucleus 22® model was gradually replaced by the model Nucleus 24®, also developed by Cochlear®. The Nucleus 24 (N24) is a multichannel cochlear implant, consisting of an internal component (composed of receiver / stimulator, beam with 24 electrodes and two extra-cochlear electrodes, used in different modes of stimulation), two different models of speech processor (model SPrint in the format of 'box' used by the user, and Esprit model, the format processor retroauricular), and transmitting antenna.
The N24 has the option of different speech processing strategies: SPEAK, Continuous Interleaved Sample (CIS) and Advanced Encoder Conversion (ACE).
The ACE strategy combines the advantages of the SPEAK and CIS strategies. The frequency spectrum is divided into 22 channels for stimulation. The number of selected peak is constant and depends on the value specified in programming the speech processor. Just as the CIS, the ACE strategy has a higher rate of stimulation as compared to SPEAK, ranging between 500 and 2400Hz (8).
WALTZMAN et al., 1999 (9) conducted a study comparing the performance of cochlear implant users N22 and N24, in tests of speech perception presented in open format. The authors used 16 users, N22, N24 and 20 users; all with the strategy SPEAK speech coding. The average age of users of N22 was higher than the N24, as well as time of deprivation hearing. They concluded that speech perception by users of N22 and N24 is similar to the three months of use, after three months, users of N22 showed no significant changes in their performance, while users of N24 continued to evolve. The authors stress the importance of further studies, especially comparing different forms of stimulation and speech coding strategies.
BRITO, 2000 (2) studied the hearing results and quality of life of 10 cochlear implant patients using the N22 model. After six months of use, with a strategy for speech processing SPEAK, patients showed an average of audiometric thresholds at 42.7 dB HL, 82.8% accuracy in the recognition test sentences in presentation in zipped format, and 56% Hit in the presentation of sentences in an open format. The questionnaire of quality of life after implantation showed an improvement in perceived quality of life for families of users of CI.
BENTO et al., 2004 (1) reviewed the hearing results in 61 cochlear implant users, with a minimum of six months of use. Mean audiometric thresholds was 38.7 SPL, while the average in recognition of sentences in an open format was 71.3%, 86.5% of vowels, monosyllables at 52.60% and 52.6% in medial consonant. Most patients were able to use the phone. The authors concluded that the patients obtained excellent results in tests of recognizing words and sentences in open presentation, regaining a useful hearing. However, there was the concern of separation technologies that work.
Our objective in this study to check for the contribution of technology on N24 audiometric thresholds and performance in the perception of sentences when compared to the N22, after 12 months of use.
METHOD Procedures
This is a retrospective cross-sectional sample in the database, held at the Training Program in the Cochlear Implant Cochlear Implant Clinic at the Hospital das Clinicas, Faculty of Medicine, University of São Paulo (FMUSP), having been authorized by the responsible entity and approved by the Institutional Ethics Committee under protocol 633/04.
The criteria for sample selection were:
- Aged 18 years of age when a person is considered adult (10).
- Use of device models Cochlear Implant Nucleus 22 or Nucleus 24 (Cochlear Ltd - Australia.)
- Systematic use of IC (use for at least eight hours daily for 12 consecutive months)
- Data equivalent to perception test sentences and audiometric thresholds in the period of 12 months of CI use.
Of the patients selected according to the above criteria, we excluded patients:
- Etiology of hearing loss is meningitis.
- With time of deprivation hearing not less than 20 years.
We analyzed data from medical records of all patients undergoing cochlear implant surgery at the institution operated from January 1999 to May 2007. The charts were selected according to age, and analyzed in two groups according to the model of IC: Group 22 (G22) - N22 Users and Group 24 (G24) - N24 users. The groups were analyzed in subgroups according to duration of auditory deprivation: from zero to 10 years and 11-20 years.
Data were collected from the preoperative evaluation and the IC after 12 months of use of audiometric thresholds in free field at frequencies of 500Hz, 1kHz, 2kHz, 4kHz, 6kHz and 8kHz, and test results of the perception of sentences presented in open and closed in a silent environment (11). The thresholds were considered as missing 130dB.
The perception test sentences in open presentation are performed by ear, without the support of gestures or lip reading. The test carried out in closed presentation is also done by ear, but the patient has the visual support in written multiple-choice sentences that will be used. These tests are based on the Protocol Latin American (12) for evaluation of the IC, and are part of the Protocol HCFMUSP (3).
The groups were compared to results in the perception test sentences and mean audiometric thresholds. To compare these variables between the groups G22 and G24, we used the nonparametric Mann-Whitney. To compare the patients' age and time of deprivation, we used the Student t test for independent samples. Were considered statistically significant when p values <0.05.
RESULTS Of the 84 adult patients with meningitis etiology non-deployed with the N22 and N24 models, 54 patients met the selection criteria, 13 and 41 users of N22 to N24 users.
The duration of auditory deprivation between groups was statistically similar, as are the ages of users of the G22 and G24 (Table 1).
There was no statistically significant difference between preoperative residual hearing from users of IC N22 and N24 (Figure 1).
In audiometric thresholds after a year of CI use, a statistically significant difference between the groups G22 and G24, G24 and the thresholds of better than the same variable in G22 (Figure 2).
In tests of perception of sentences in quiet, no statistically significant difference between G22 and G24 presentation in open and closed (Figure 3).
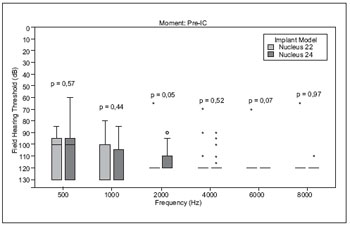
Figure 1. Comparison between threshold pre-implant users' groups G22 and G24.
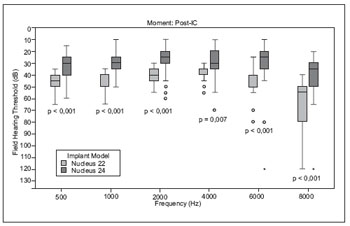
Figure 2. Comparison of the audiometric thresholds post-implant users among the groups G22 and G24.
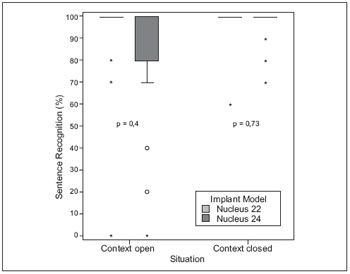
Figure 3. Comparison of performance on tests of perception of sentences in quiet environment between users groups G22 and G24.
Our sample included 54 subjects, 13 users and 41 users N22 N24. This difference in the sample due to the N22 that was used in 1999, 2000, 2001, being replaced by N24 from 2001.
We excluded patients whose etiology of hearing loss was meningitis, since this is considered a predictor for a poor performance with the CI due to the reduced number of spiral ganglion cells, a characteristic lesion in the auditory system caused by meningitis (4,13).
We selected patients whose time of deprivation hearing was between zero and 20 years, thus avoiding this variable in the results. Some authors suggest that a long period of auditory deprivation can affect the tests of speech perception (9,13,14, 15). But the era of auditory deprivation between the two groups were very homogeneous, and therefore did not affect the variables.
Clinically, we observe the contribution of technology on the N24 the N22. However, it is necessary to confirm this contribution, since it directly affects the indications of cochlear implant.
Our results showed a statistically significant difference in the audiometry of users of N22 and N24, and the average frequency of 500 to 4000Hz, 6000Hz and 8000Hz were of lower in users of N24, showing better performance in audiometry. These results corroborate the literature (16), which also reported better audiometric thresholds in users of N24 compared to N22. The contribution of auditory thresholds may have implication in the perception of sounds of low acoustic energy and low intensities (17,18).
At the same time, it was important to the homogeneity of the samples with respect to residual hearing preoperatively. As the audiological criteria are evolving with technology (13,19), there would doubt that the patients in G24 could be favored by higher residual preoperative.
SKINNER et al. (1999b) (17) evaluated the speech perception of eight post-lingual adults, users of IC N22 with the SPEAK strategy, according to the minimum levels of stimulation. The minimum levels were set at a threshold (default value care), and higher levels (about +2.04 dB) to determine if the increased levels would improve the understanding of the sounds. Users of IC N22 participated in the survey carried out in four phases. The speech perception was assessed with words of core consonant-vowel-consonant in quiet, and sentences in noise, both presented at 50, 60 and 70 dB SPL during two weekly sessions at the end of each phase. The participants' average score was higher during the largest amount of words and phonemes, shown at 50 and 60 dB SPL, and presented in sentences of 50 to 70dB SPL. All participants chose to use the program with higher levels in daily life, the end of the study. The results suggest that clinical use of a program with higher levels in N22 facilitates understanding of the sounds in everyday life. More studies are needed to determine whether the approach is suitable for other models of cochlear implants.
In the perception of sentences, there were no statistically significant differences between users of N22 and N24, but the average performance of the N22 users was higher than users of the N24. WALTZMAN et al., 1999 (8) concluded that speech perception in users of the N22 and N24 is similar, but believed that after 12 months of use, the technology could benefit them in speech perception.
In our sample, the median of the G22 in the perception test sentences was already 100%, i.e., the good performance of the patients influenced the statistical outcome. The application of the most difficult tests, such as compound words for 'vowel-consonant-vowel', could change the performance of patients.
Users of N22 in our sample used the strategy of the SPEAK speech processing, while all users of the N24 used the strategy of ACE speech processing.
PSARROS et al., 2002 (18) compared the performance of seven children users of N24 during the transition from using the SPEAK strategy, used by at least six months for the ACE strategy. In the study, the biggest difference is established between the strategies was the speed of stimulation (250Hz for SPEAK, and ACE to 900Hz). After the shift, no deterioration in speech perception, but some children had more difficulty at the beginning of using the ACE strategy. The authors concluded that there was an improvement in speech perception of children, and that patients can benefit by switching the two speech coding strategies in different listening situations.
SKINNER et al., 2002 (20) compared the performance of 62 subjects, users of the strategies SPEAK, ACE and CIS, all N24 users, through the application of lists of words, sentences, test medial consonant and vowel. Users experienced different strategies, and the authors concluded that the ACE strategy performed better on tests of speech perception.
ROMERO et al., 2004 (21) studied outcomes observed in patients post-lingual cochlear implant users, reported that between six and 12 months of cochlear implant use, some patients obtain maximum performance during this period, while others continue to evolve after the 12 months of use.
Regarding the influence of duration of auditory deprivation on speech perception, we found no statistically significant differences between groups divided according to the time of deprivation. The users of the N22, performance in the perception test sentences in closed set was similar, regardless of time of auditory deprivation, disagreeing with the findings reported in the literature (13,15). These authors reported that the duration of auditory deprivation is directly proportional to lower performance in speech perception.
In our study, all speech perception tests were applied in a state of silence. Noisy situations are more challenging for cochlear implant user since they reduce significantly the rate of speech recognition. However, the training of listening skills and knowledge of strategies to minimize the negative effects of noise on communication are extremely important (22). Most studies of speech perception were performed in quiet situations (23). This study led us to choose assessment protocols that include testing and challenging situations that reflect the daily lives of patients.
BRITO NETO, 2000 (2) noted that while the hearing evaluation is an objective criterion for evaluating the patient before and after cochlear implant, she alone did not reflect the outcome of the condition of the listener to patients before the deaf. The socio-economic and cultural needs of patients implanted and thus their different needs for use of hearing necessitate an evaluation that takes into account the situations and environments acoustic characteristics of their lifestyle and their relationship with family and community.
CONCLUSION Users of IC N24 had the best averages in audiometric thresholds when compared to users of the N22, but the speech perception tests performed indicated no difference between the models. There was no difference between the tests of perception of sentences and audiometric thresholds are related to the time of deprivation hearing, except at the threshold of 8000Hz in users of N22. When the intention is to determine the contribution of technology, studies should be conducted with larger samples and tests in challenging situations (eg, speech in noise), to verify that the technology also influences the results in speech perception.
BIBLIOGRAPHICAL REFERENCES1. Bento RF, Brito RV, Castilho AM, Goffi Gomez MVS, Giorgi SB, Guedes MC. Resultados auditivos com o implante coclear multicanal em pacientes submetidos a cirurgia no Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. Rev Bras Otorrinolaringol. 2004, 70(5):632-7.
2. Brito RV. Estudo dos resultados auditivos e da qualidade de vida em pacientes com implante coclear multicanal. São Paulo, 2000. (Tese de Doutorado - Faculdade de Medicina da Universidade de São Paulo).
3. Goffi-Gomez MVS, Guedes MC, Sant'Anna SBG, Peralta CGO, Tsuji RK, Castilho AM, Brito RV, Bento RF. Critérios de seleção e avaliação médica e audiológica dos candidatos ao implante coclear: Protocolo HCFMUSP. Arq Otorrinolaringol. 2004, 7(3):197-204.
4. Guedes MC, Brito RV, Goffi-Gomez MVS, Sant'Anna SBG, Peralta CGO, Castilho AM, Bento RF. Telemetria de resposta neural intra-operatória em usuários de implante coclear. Rev Bras Otorrinolaringol. 2005, 71(5):660-7.
5. Clark GM, Tong YC, Bailey QR, Black RC, Martin LF, Milar JB, O'Loughlin BJ, Patrick JF, Pyman BC. A multiple-electrode cochlear implant. J Oto-Laryngol Soc. Aust. 1978, 4:208-212.
6. Skinner MW, Fourakis MS, Holden TA, Holden LK, Demorest ME. Identification of speech by cochlear implant recipients with the multipeak (MPEAK) and spectral peak (SPEAK) speech coding strategies II. Consonants. Ear and Hear. 1999, 20(6):443-60.
7. Skinner MW, Holden LK, Holden TA, Demorest ME. Effect of stimulation rate on cochlear implant recipients' thresholds and maximum acceptable loudness levels. J Am Acad Audiol. 2000, 11(4):203-13.
8. Skinner MW, Holden LK, Whitford LA, Plant KL, Psarros C, Holden TA. Speech recognition with the Nucleus 24 SPEAK, ACE and CIS speech coding strategies in newly implanted adults. Ear Hear. 2002, 23:207-223.
9. Waltzman SB, Cohen NL, Roland JT. A comparison of the growth of open-set speech perception between the Nucleus 22 and Nucleus 24 cochlear implant systems. The American J Otol. 1999, 20:435-41.
10. Brasil, Casa Civil, 2003. Estatuto do Idoso. Disponível em: http://www.planalto.gov.br/ccivil/LEIS/2003/L10.741.htm.
11. Costa MJ, Iorio MCM, Mangabeira-Albernaz PL. Desenvolvimento de um teste para avaliar a habilidade de reconhecer a fala no silêncio e no ruído. Pró-fono. 2000, 12(2):09-16.
12. Protocolo Latino Americano para Avaliação de candidatos ao Implante Coclear. Reunião do Grupo de Investigação Latino-Americano da Cochlear Corporation. Bogotá, Colômbia, 2000.
13. Green KMJ, Bhatt YM, Mawman DJ, O'Driscoll MP, Saeed SR, Ramsden RT, Green MW. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007, 8(1):1-11.
14. Lyxell B, Andersson J, Arlinger S, Bredberg G, Harder H, Rönnberg J. Verbal information-processing capabilities and cochlear implants: implications for preoperative predictors of speech understanding. J Deaf Stud Deaf Education 1:3 Summer, 1996.
15. Fallon JB, Irvine DRF, Sheperd RK. Cochlear implants and brain plasticity. Hear Res. 2008, 238:110-17.
16. Goffi-Gomez MVS, Guedes MC, Peralta CGO, Tsuji RK, Neto RVB, Bento RF. Limiares auditivos em campo livre em usuários de implante coclear Nucleus 22 e Nucleus 24. Anais do VI Congresso da Fundação Otorrinolaringologia - FORL, 2007.
17. Skinner MW, Holden LK, Holden TA, Demorest ME. Comparison of two methods for selecting minimum stimulation levels used in programming the Nucleus 22 cochlear implant. J Speech Lang Hear Res. 1999, 42(4):814-28.
18. Psarros CE, Plant KL, Lee K, Decker JA, Whitford LA, Cowan RSC. Conversion from the SPEAK to the ACE strategy in children using the Nucleus 24 cochlear implant system: speech perception and speech production outcomes. Ear Hear. 2002, 23:18S-27S.
19. David EE, Ostroff JM, Shipp D, Nedzelski JM, Chen JM, Parnes LS, Zimmerman K, Schramm D, Seguin C. Speech coding strategies and revised cochlear implant candidacy: an analysis of post-implant performance. Otol Neurotol. 2003, 24(2):228-33.
20. Skinner MW, Arndt PL, Staller SJ. Nucleus 24 Advanced Encoder Conversion study: performance versus preference. Ear Hear. 2002, 23:2S-17S.
21. Romero MJP, Quevedo MS, Segura CR. Cochlear implant in postlingual adults with progressive hearing loss. Acta Otorrinolaringol Esp. 2004, 55:457-462.
22. Nascimento LT, Bevilacqua MC. Avaliação da percepção da fala com ruído competitivo em adultos com implante coclear. Rev Bras Otorrinolaringol. 2005, 71(4):432-8.
23. Bishop R, Littman T, Balko K, Watson S, Backous D. Speech understanding in noise with post-lingual adult cochlear implant users: a comparison of devices. Cochlear Impl Int. 2003, 4(supplement 1):4-5.
1 Fellow in Cochlear Implant, Hospital das Clinicas, Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP. Speech Cochlear Implant Team at the Hospital das Clinicas, Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP.
2 PhD in Human Communication Disorders (Speech Pathology) from the Federal University of São Paulo. Speech; Hospital of the Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP.
3 Free Teaching from the University of São Paulo. Speech Therapist, Associate Professor, University of São Paulo.
4 Doctorate in Human Communication Disorders at the Federal University of São Paulo. Speech Therapist, Associate Professor, University of São Paulo.
5 Doctor of Health Sciences, University of São Paulo. ENT, Hospital of the Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP.
6 Free Teaching Hospital of the Faculty of Medicine, University of São Paulo, HC-FMUSP. ENT, Hospital of the Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP.
7 Free Teaching from the University of São Paulo. ENT, Hospital of the Faculty of Medicine, University of São Paulo, HC-FMUSP, São Paulo, SP.
Institution: Training Program in Cochlear Implant Department of Physical Therapy, Speech Therapy and Occupational Therapy, University of São Paulo, and the Cochlear Implant Team at the Hospital das Clinicas, Faculty of Medicine, University of São Paulo. São Paulo / SP - Brazil. Mail Adress: Paola Angelica Samuel - Rua Capote Valente, 432 - Conjunto 14 - Pinheiros - São Paulo / SP - Brazil - Zip code: 05409-001 - Telephone: (+55 11) 3898-2210 - E-mail: paolasamuel@yahoo .com.br
Article received on April 22, 2010. Article accepted on June 10, 2010.