 |
409 |
|
| Year: 2007 Vol. 11 Num. 1 - Jan/Mar - (11º)
|
|
 |
|
|
| Nasal Myiasis: Case Report and Literature Review |
|
| Author(s): |
Alberto Marcos Manfrim1, Alexandre Cury2, Pedro Demeneghi3, Geraldo Jotz4, Renato Roithmann5
|
|
|
| Key words: |
| Myiasis. Nasal obstruction. Endoscopic surgery. |
|
|
 |
| Abstract: |
Introduction: Nasal myiasis isn´t a frequent patology and happen mainly in development countries and in the summer. Objective: To report a case of a patient with a nasal malignant tumor that developed myiasis. Case Report: Seventy year-old female patient presenting malignant nasal neoplasia and myiasis, the treatment required specific drug, ivermectin, plus functional endoscopic sinus surgery. Critical aspects related to the diagnosis and treatment of nasal myiasis are revised. Conclusion: Nasal myiais can request to be treat with drug and surgery, and is important to reduce the risk factors to myiasis.
|
|
 |
INTRODUCTION
Myiasis has been defined as an infestation on humans and vertebrate animals by larvae of insects, which feed themselves, for certain time, on living or dead tissues from the host or on fluid substance(1). The presence of larvae in the nasal cavity is called nasal myiasis, which, although uncommon(2), is an embarrassing situation for the patient, family and responsible team(3). Most of the cases occur in developing countries where sanitation is a public health problem. The most common cases occur during summer and in tropical climate areas due to the fact that the larvae need warm temperature to unincubate.
Myiasis from some types of larvae can be useful to the host because they help on necrotic tissue extraction. That is why larvae were used to therapeutic removal of wounds (4). Therefore, such larvae can lead to more complicated situations when they infect areas such as eyes, nose and ears.
The target of this study is to report a clinical case of nasal myiasis on an elderly patient, suffering from nasal cavity and paranasal sinus tumor and developed favorably with clinical therapy and surgery and also to review the literature on the topic.
CASE REPORT
T.G., 70 years old, female, Caucasian, retired (former nurse), catholic, single, high school degree from Porto Alegre - RS. Patient came to see a doctor complaining of nasal bleeding at the left initially 15 days ago. She presented permanent nasal obstruction, nose itching and sneezes. In her clinical report, there was evidence of 4 resections of basal cell carcinoma in nasolabial area. The first incidence was 26 years ago and the second 3 yeas ago, and when this one occurred part of the left hard palate was also resected due to tumor infiltration. After the third surgery, which was performed at another service, radiotherapy treatment was recommended causing sight loss at the left eye. In her psychosocial history, it was attested that patient lives in nursing home where there are a large amount of flies. The physical exam showed the patient's general condition was regular; she weighed 47kg, walked only with somebody's help; her left sight had a deviation of the middle line and her cornea was obscure. In the oroscopy, her hard palate presented 3cm-diameter perforation at the left, connecting with nasal cavity and covered by serosanguineous secretion. Previous rhinoscopy showed right nasal fossa totally closed by nasal septum deviation and in the nasal fossa it was observed ulceration on nasal entrance with bleeding in small quantity. Besides, a great quantity of larvae causing obstruction of the nasal fossa and making visualization of nasal concha difficult, and moreover, superior and inferior side cartilage were exposed and partially destroyed (Picture 1) After diagnosing myiasis on the left nasal fossa, some of the visible larvae were removed and then introduced ivermectin 6mg once a day, taken orally.
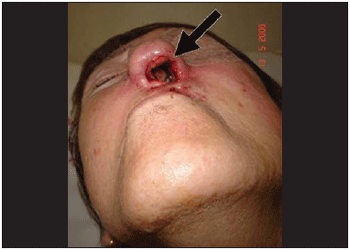 Picture 1. External aspect of the nose. It is noticed the extensive area of necrosis on left nasal vestibule with skin erosion.
On the following day, patient was hospitalized and, under general anesthesia, underwent to surgery through videoendoscopy in order to clean nasal cavity (Picture 2). In this procedure, ether was used in order to make larvae removal easier, though with no expressive difference on their vigor. The collected material was examined in anatomopathological terms, which revealed invasive carcinoma slightly ulcerated different with extensive areas of necrosis on respiratory mucosa.
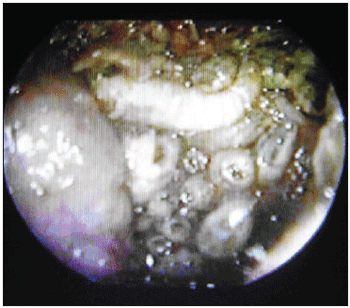 Picture 2. Larvae on the left nasal cavity.
It was performed exams such as CT and nuclear magnetic resonance imaging of the paranasal sinus, orbits and cranial base (Pictures 3, 4 and 5). They dismissed intracranial invasion, though showed implication of the whole paranasal sinus by soft material, then there was no more need to inform they were larvae, polyps or secretion.
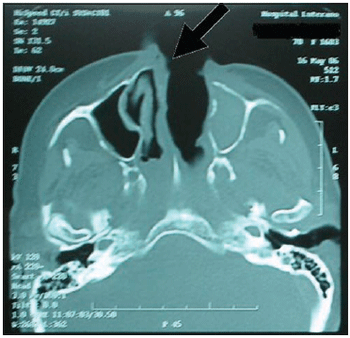 Picture 3. CT of the paranasal sinus - axial cut: it is noticed obstruction on right nasal fossa by displacement of nasal septum. 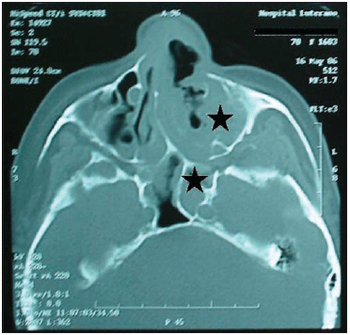 Picture 4. CT of the paranasal sinus - axial cut: it is noticed comprehensive covering of the left maxillary sinus with protection of bone walls and also implication of the left anterior and posterior ethmoid and sphenoid sinus. 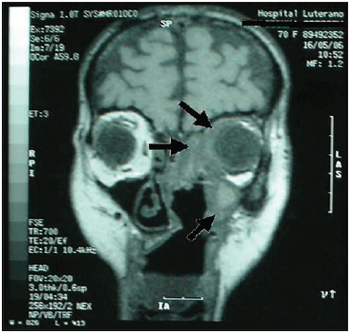 Picture 5. Magnetic resonance imaging of the skull and paranasal sinus - coronal cut: it is noticed implication on periorbitary tissues to the left; absence of intracranial invasion or implication of ethmoidal and maxillary sinus to the left.
After three days of ivermectin use, there was still the presence of living larvae in the left nasal fossa. So another surgery was performed through videoendoscopy in order to totally clean the affected paranasal sinus. Then it was performed maxillary antrostomy ethmoidectomy sphenoidectomy and frontal left sinusotomy. During such procedure several dead larvae and polyplike tissues of the maxillary sinus and left ethmoid were removed (Picture 6). In the other paranasal sinus it was only observed purulent secretion and necrotic tissues.
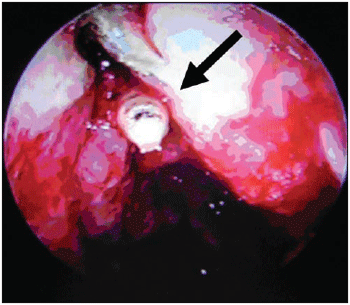 Picture 6. Larvae after left anterior ethmoid opening (transoperative).
The histological exam revealed invasive carcinoma of respiratory mucosa slightly different. In the following 4 days no more larvae were noticed and symptoms of nasal obstruction, itching, sneezes and bleeding occurred. On the fifth day after surgery, patient was released from hospital.
DISCUSSION
This study reports a diagnoses and management of a nasal myiasis case, affecting an elderly patient with recurrence of malignant nasal tumor, which was solved through clinical and surgical therapy. It focuses on the difficulty in removing all larvae from the nasal cavity without surgery of paranasal sinus and also the possibility of resistance of the larvae regarding the use of inverctin.
The revision of the proper amount of inverctin use for myiasis cases just shows a study in which the quantity is of 200µg/Kg(5) in only one dose. For the current patient, it was used a dose of 127 µg/Kg/day and surgical approaches showed the presence of living larvae.
Myiasis can be classified into two types: Furuncular Myiasis and secondary myiasis(6). The former is caused by larvae of fly Dermatobia hominis, which penetrated the tissue causing painful inflammatory nodules, with fistulazation. After larvae achieve their maturation in the subcutaneous tissue, they spontaneously leave the host. Diagnosis is performed by the examination of the inflammatory nodules similar to furuncles, with drainage of serosanguineous secretion, pain and perception of movements of the larvae end in the fistulous opening.
Secondary myiasis (of open cavities or wounds) is popular known as "bicheira". There are several larvae of flies in places where there is loss of integrity of the skin, scale, feather, etc. They are usually caused by flies Callitroga, Lucilia and Musca. The typical clinical aspect is a great number of larvae moving locally.
Flies deposit their eggs in the host, emerging from 8 to 24 hours later depending on the species and temperature. Larvae will feed themselves of infected or dead tissues and secretion during some days up to get grown. After that, they leave the host and carry on developing in an isolated place, such as under a piece of furniture. The larva remains still, its exoskeleton gets dark, and then it begins its pupal stage (Picture 7). From this moment it undergoes an important physical change, emerging as an adult fly from 1 to 3 weeks later. The lasting period of each phase depends on the fly. Young larvae do not multiply, only adult ones do.
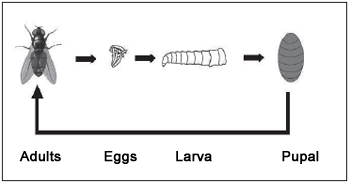 Picture 7. Typical Life cycle of a fly.
Sharma et al(3) says the disease occurs equally for both men and women and usually attacks people over 50 and mainly the ones of low social class. Patients usually present epistaxis, nasal obstruction, rhinorrhea, fetid nasal smell, facial pain and headache.
Rao(7) reported three cases of nasal myiasis and observed that nasal ulcers and rhinorrhea, which emanate bad smell, attract flies, so they leave their eggs while patients sleep. Sood et al(8) showed that atrophic rhinitis is a risky factor, though, besides reducing patient nasal sensitivity and increasing nasal cavity, it produces fetid smell and drainage of purulent thick secretion.
Rhinoscopy usually shows edematous mucosa, with mucus full of necrotic material and larvae. This causes indisposition to patient. Larvae can diffuse to paranasal sinus, lacrimonasal duct, orbit, face skin and occasionally intracranial structures leading to meningitis. They eat anything. Bone is usually destroyed by larvae and then the infection can cause osteomyelitis. They can also ruin face and eyes(3). Thus, previous diagnosis is essential to limit tissue destruction and associated complications. In order to trace the severity of the disease and decide which surgical procedure should be taken, it is necessary imaging(9) studies.
To prevent myiasis, it is necessary: to reduce risky factors by making the host less attractive and reduce the number of flies in the environment. Patients with drainage or chronic ulcers are more sensitive to myiasis. Dirty clothes, body fluid or purulent drainage are highly attractive to flies which cause myiasis. Wounds and drainage openings or fetid smell should remain clean and covered all the time. Reducing the number of flies is not so easy. It requires integrated participation by multiple services and clean places.
The well-being of patient is the priority of myiasis therapy(10). In the cases of nasal myiasis, potency of respiratory pathway needs to be assured. The comprehensive respiratory implication can result from a physical blockage by the presence of larvae or by bleeding on the air pathway.
The aim of nasal myiasis treatment as well as other secondary myiasis is to remove all invader organisms. Removing larvae from nasal cavity id not a simple procedure, especially for the difficulty of visualize larvae directly and worst of all, there are few health volunteers to do this job. In order to visualize larvae, nasal endoscope has been used. This has been helping on finding and removing larvae from inaccessible areas(11). In the current case, endoscopy helped positively on the exploration and surgical drainage of all paranasal sinuses.
Several substances are used in order to make removal of larvae easy. They are: mixture of chloroform and turpentine (1:4)(3), ethylene chloride, irrigation on the area with naphtha, ether and cocaine(9), ivermectin at 1%(13), and lidocaine. Ivermectin by oral use has been described in the literature for myiasis therapy. Only one dose of 200µg/Kg is taken, obtaining result in 48 hours(5).
It is important to point that immunoprophylaxis to tetanus should be updated if necessary, and antibiotics should be prescribed when there is signs of associated bacteria infection.
CONCLUSION
Nasal myiasis, many times, besides specific therapy to eliminate the larvae, also requires surgery approach to comprehensive removal of the paranasal sinus. Endoscopic surgery helps on visualization and surgery in such cases. Preventive procedures are also important in order to improve care with necrotic wounds and with environment where patient lives.
REFERENCES
1. Hope FW. On insects and their larvae occasionally found in the human body. Trans R Entomol Soc London 1840, 2:256-271.
2. Quesada P, Navarrete ML, Maeso J. Nasal myiasis due to Oestrus ovis larvae. Eur Arch Otorhinolaryngol; 1990, 247(2):131-2.
3. Sharma H., Dayal D., Agrawal S.P. Nasal myiasis: Review of 10 years experience. The Journal of Laryngology and Otology; 1989, 103:489-491.
4. Young T. Maggot therapy in wound management. Community Nurse; 1997, 3:43-45.
5. De Tarso P, Pierre-Filho P, Minguini N, Pierre LM, Pierre AM. Use of ivermectin in the treatment of orbital myiasis caused by Cochliomyia hominivorax. Scand J Infect Dis.; 2004, 36(6-7):503-5.
6. Bakos L. Zoodermatose. In: Duncam BB. Medicina Ambulatorial: Condutas de Atenção Primária Baseadas em Evidências. 3º Edição - Porto Alegre - ArtMed; 2004, p. 1033-34.
7. Rao G.R. Myiasis in lepers. Indian Medical Gazette, 1929, 64: 308-382.
8. Sood, V.P., Kakar P.K., Wattal B.L. Myiasis in otorhinolaryngology with entomological aspects. Journal of Laryngology and Otology., 1976, 90:393-399.
9. Lui PC., Lee MK., Wong JH., Leung CY., Lee CK., Lai RW., Lam ET., Yeung JC., Suen DT., Tse GM. Myiasis by chrysomya bezziana in surgical pathology. Pathology., 2005, 37(1):80-82.
10. Sherman RA, Roselle G, Bills C, Danko LH, Eldridge N. Healthcare-associated myiasis: prevention and intervention. Infect Control Hosp Epidemiol; 2005, 26(10):828-32.
11. Soni NK. Endoscopy in nasal myiasis. Trop Doct. 2000, 30(4):225-227.
12. Ford T, Anania WC, Rosen RC, Mirarchi J, Liaw WH. Treatment of cutaneous myiasis of lower extremity ulcerations with ethyl chloride. J Am Pediatr Med Assoc; 1986, 76:690-692.
13. Victoria J, Trujillo R, Barreto M. Myiasis: a successful treatment with topical ivermectin. Int J Dermatol; 1999, 38(2):142-4.
14. Masoodi M, Hosseini K. The respiratory and allergic manifestations of human myiasis caused by larvae of the sheep bot fly (Oestrus ovis): a report of 33 pharyngeal cases from southern Iran. Ann Trop Med Parasitol; 2003, 97(1):75-81.
1. Medical Resident of the Year 1 of the Department of Otorhinolaryngology, ULBRA's Hospital Complex.
2. Medical Resident of 3 years of service Otorhinolaryngology, ULBRA's Hospital Complex.
3. Medical Resident of 2 years of service Otorhinolaryngology, ULBRA's Hospital Complex.
4. Otorhinolaryngologist. Adjunct Professor of Otolaryngology and Head and Neck Surgery of the ULBRA and the Department of Science Morfológicas, UFRGS. Post-Doctorate in Otolaryngology at the University of Pittsburgh.
5. Otorhinolaryngologist. Adjunct Professor of Otolaryngology and Head and Neck Surgery of the ULBRA. Professor of Post-Graduate Medical Clinic in, UFRGS.
Insituição: Lutheran University of Brazil (ULBRA).
Alberto Marcos Manfrim
Address to correspondênica: Street Venâncio Aires, 1416 - Neighborhood Niteroi - CEP 92110-000 - Canoes / RS - Fax (51) 3475-7542 -- E-mail: albertomanfrim@hotmail.com
This article was submitted in Management System Publications in the R@IO 9/7/2006 and approved on 19/12/2006 21:51:36
|
|
 |
|
|
